Early afterdepolarizations promote transmural reentry in ischemic human ventricles with reduced repolarization reserve
- PMID: 26850675
- PMCID: PMC4821233
- DOI: 10.1016/j.pbiomolbio.2016.01.008
Early afterdepolarizations promote transmural reentry in ischemic human ventricles with reduced repolarization reserve
Abstract
Aims: Acute ischemia is a major cause of sudden arrhythmic death, further promoted by potassium current blockers. Macro-reentry around the ischemic region and early afterdepolarizations (EADs) caused by electrotonic current have been suggested as potential mechanisms in animal and isolated cell studies. However, ventricular and human-specific arrhythmia mechanisms and their modulation by repolarization reserve remain unclear. The goal of this paper is to unravel multiscale mechanisms underlying the modulation of arrhythmic risk by potassium current (IKr) block in human ventricles with acute regional ischemia.
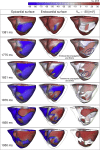
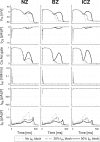
Methods and results: A human ventricular biophysically-detailed model, with acute regional ischemia is constructed by integrating experimental knowledge on the electrophysiological ionic alterations caused by coronary occlusion. Arrhythmic risk is evaluated by determining the vulnerable window (VW) for reentry following ectopy at the ischemic border zone. Macro-reentry around the ischemic region is the main reentrant mechanism in the ischemic human ventricle with increased repolarization reserve due to the ATP-sensitive potassium current (IK(ATP)) activation. Prolongation of refractoriness by 4% caused by 30% IKr reduction counteracts the establishment of macro-reentry and reduces the VW for reentry (by 23.5%). However, a further decrease in repolarization reserve (50% IKr reduction) is less anti-arrhythmic despite further prolongation of refractoriness. This is due to the establishment of transmural reentry enabled by electrotonically-triggered EADs in the ischemic border zone. EADs are produced by L-type calcium current (ICaL) reactivation due to prolonged low amplitude electrotonic current injected during the repolarization phase.
Conclusions: Electrotonically-triggered EADs are identified as a potential mechanism facilitating intramural reentry in a regionally-ischemic human ventricles model with reduced repolarization reserve.
Keywords: Computer-based model; Ischemic heart disease; Potassium channels; Repolarization; Ventricular arrhythmia.
Copyright © 2016 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures






Similar articles
-
Effects of early afterdepolarizations on reentry in cardiac tissue: a simulation study.Am J Physiol Heart Circ Physiol. 2007 Jun;292(6):H3089-102. doi: 10.1152/ajpheart.01309.2006. Epub 2007 Feb 16. Am J Physiol Heart Circ Physiol. 2007. PMID: 17307992
-
High arrhythmic risk in antero-septal acute myocardial ischemia is explained by increased transmural reentry occurrence.Sci Rep. 2019 Nov 14;9(1):16803. doi: 10.1038/s41598-019-53221-2. Sci Rep. 2019. PMID: 31728039 Free PMC article.
-
Effects of L-type calcium channel and human ether-a-go-go related gene blockers on the electrical activity of the human heart: a simulation study.Europace. 2015 Feb;17(2):326-33. doi: 10.1093/europace/euu122. Epub 2014 Sep 15. Europace. 2015. PMID: 25228500 Free PMC article.
-
Ionic mechanisms of ischemia-related ventricular arrhythmias.Clin Cardiol. 1996 Apr;19(4):325-31. doi: 10.1002/clc.4960190409. Clin Cardiol. 1996. PMID: 8706374 Review.
-
Early afterdepolarizations in cardiac myocytes: beyond reduced repolarization reserve.Cardiovasc Res. 2013 Jul 1;99(1):6-15. doi: 10.1093/cvr/cvt104. Epub 2013 Apr 25. Cardiovasc Res. 2013. PMID: 23619423 Free PMC article. Review.
Cited by
-
Computational modeling of cardiac electrophysiology and arrhythmogenesis: toward clinical translation.Physiol Rev. 2024 Jul 1;104(3):1265-1333. doi: 10.1152/physrev.00017.2023. Epub 2023 Dec 28. Physiol Rev. 2024. PMID: 38153307 Free PMC article. Review.
-
Initiation of ventricular arrhythmia in the acquired long QT syndrome.Cardiovasc Res. 2023 Mar 31;119(2):465-476. doi: 10.1093/cvr/cvac103. Cardiovasc Res. 2023. PMID: 35727943 Free PMC article.
-
Altered Calcium Handling and Ventricular Arrhythmias in Acute Ischemia.Clin Med Insights Cardiol. 2016 Dec 14;10(Suppl 1):61-69. doi: 10.4137/CMC.S39706. eCollection 2016. Clin Med Insights Cardiol. 2016. PMID: 28008297 Free PMC article. Review.
-
Mechanisms of Premature Ventricular Complexes Caused by QT Prolongation.Biophys J. 2021 Jan 19;120(2):352-369. doi: 10.1016/j.bpj.2020.12.001. Epub 2020 Dec 15. Biophys J. 2021. PMID: 33333033 Free PMC article.
-
Computational models in cardiology.Nat Rev Cardiol. 2019 Feb;16(2):100-111. doi: 10.1038/s41569-018-0104-y. Nat Rev Cardiol. 2019. PMID: 30361497 Free PMC article. Review.
References
-
- Browne K.F., Prystowsky E., Heger J.J., Chilson D.A., Zipes D.P. Prolongation of the Q-T interval in man during sleep. Am. J. Cardiol. 1983;52:55–59. - PubMed
-
- Cao J.-M., Qu Z., Kim Y.-H., Wu T.-J., Garfinkel A., Weiss J.N., Karagueuzian H.S., Chen P.-S. Spatiotemporal heterogeneity in the induction of ventricular fibrillation by rapid pacing. Circ. Res. 1999;84:1318–1331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

