Individual sympathetic postganglionic neurons coinnervate myenteric ganglia and smooth muscle layers in the gastrointestinal tract of the rat
- PMID: 26850701
- PMCID: PMC4935554
- DOI: 10.1002/cne.23978
Individual sympathetic postganglionic neurons coinnervate myenteric ganglia and smooth muscle layers in the gastrointestinal tract of the rat
Abstract
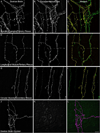
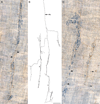
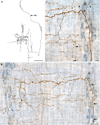
A full description of the terminal architecture of sympathetic axons innervating the gastrointestinal (GI) tract has not been available. To label sympathetic fibers projecting to the gut muscle wall, dextran biotin was injected into the celiac and superior mesenteric ganglia (CSMG) of rats. Nine days postinjection, animals were euthanized and stomachs and small intestines were processed as whole mounts (submucosa and mucosa removed) to examine CSMG efferent terminals. Myenteric neurons were counterstained with Cuprolinic Blue; catecholaminergic axons were stained immunohistochemically for tyrosine hydroxylase. Essentially all dextran-labeled axons (135 of 136 sampled) were tyrosine hydroxylase-positive. Complete postganglionic arbors (n = 154) in the muscle wall were digitized and analyzed morphometrically. Individual sympathetic axons formed complex arbors of varicose neurites within myenteric ganglia/primary plexus and, concomitantly, long rectilinear arrays of neurites within circular muscle/secondary plexus or longitudinal muscle/tertiary plexus. Very few CSMG neurons projected exclusively (i.e., ∼100% of an arbor's varicose branches) to myenteric plexus (∼2%) or smooth muscle (∼14%). With less stringent inclusion criteria (i.e., ≥85% of an axon's varicose branches), larger minorities of neurons projected predominantly to either myenteric plexus (∼13%) or smooth muscle (∼27%). The majority (i.e., ∼60%) of all individual CSMG postganglionics formed mixed, heterotypic arbors that coinnervated extensively (>15% of their varicose branches per target) both myenteric ganglia and smooth muscle. The fact that ∼87% of all sympathetics projected either extensively or even predominantly to smooth muscle, while simultaneously contacting myenteric plexus, is consistent with the view that these neurons control GI muscle directly, if not exclusively. J. Comp. Neurol. 524:2577-2603, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: RRID:AB10566286; RRID:AB_10562715; RRID:AB_2307337; RRID:AB_2313574; RRID:AB_2313713; RRID:AB_2336819; RRID:AB_572268; RRID:RGD_61109; RRID:nig-0000-10294; RRID:rid_000081; autonomic nervous system; celiac ganglion; enteric nervous system; muscularis externa; superior mesenteric ganglion.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
The Authors declare no conflict of interest.
Figures






References
-
- Baker DM, Santer RM. Image analysis of the sympathetic innervation of the myenteric plexus in the small intestine of mammalian species. Comp Biochem Physiol C. 1989;94(2):527–531. - PubMed
-
- Baluk P, Gabella G. Scanning electron microscopy of the muscle coat of the guinea-pig small intestine. Cell Tissue Res. 1987;250(3):551–561. - PubMed
-
- Bar-Shai A, Maayan C, Vromen A, Udassin R, Nissan A, Freund HR, Hanani M. Decreased density of ganglia and neurons in the myenteric plexus of familial dysautonomia patients. J Neurol Sci. 2004;220(1–2):89–94. - PubMed
-
- Bennett MR. Autonomic neuromuscular transmission. viii. Cambridge Eng.: University Press; 1972. p. 273.
-
- Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl) 1997;195(2):183–191. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

