A Validated Stability-Indicating and Stereoselective HPLC Method for the Determination of Lenalidomide Enantiomers in Bulk Form and Capsules
- PMID: 26850732
- PMCID: PMC4890444
- DOI: 10.1093/chromsci/bmv247
A Validated Stability-Indicating and Stereoselective HPLC Method for the Determination of Lenalidomide Enantiomers in Bulk Form and Capsules
Abstract
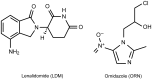
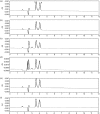
A simple, rapid and stability-indicating chiral HPLC (CHR-HPLC) method was designed for the enantiomeric separation of lenalidomide (LDM) in the presence of its degradation products. LDM was exposed to different accelerated stress factors. The degradation products were well resolved from the pure drug enantiomers. Separation of the LDM enantiomers was achieved on a LUX 5U cellulose-2 chiral column (250 × 4.6 mm i.d.) with a mobile phase consisting of methanol : glacial acetic acid : triethyl amine (100 : 0.01 : 0.01, v/v/v) at a flow rate of 1.2 mL/min. The detection wavelength was 220 nm, and ornidazole was the internal standard. The chiral method was validated in terms of its specificity, linearity, range, precision and accuracy as well as solution stability, robustness, limit of detection and limit of quantification. The calibration curve was linear for concentrations ranging from 2 to 1,000 ng/mL (r= 0.9999) for both LDM enantiomers. The proposed method, which met International Conference on Harmonization/Food and Drug Administration regulatory requirements, was utilized successfully for the determination of LDM in bulk and in capsules with acceptable accuracy and precision; the label demand percentages were 100.09 ± 0.80 and 99.97 ± 0.93 for the S-(-) and R-(+)-LDM enantiomers, respectively. Based on these results, this method should have great value when applied to quality control and stability studies of LDM.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
HPLC separation technique for analysis of bufuralol enantiomers in plasma and pharmaceutical formulations using a vancomycin chiral stationary phase and UV detection.J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Sep 1;856(1-2):328-36. doi: 10.1016/j.jchromb.2007.06.021. Epub 2007 Jun 29. J Chromatogr B Analyt Technol Biomed Life Sci. 2007. PMID: 17681871
-
Separation and determination of clenbuterol by HPLC using a vancomycin chiral stationary phase.J AOAC Int. 2009 May-Jun;92(3):824-9. J AOAC Int. 2009. PMID: 19610374
-
Enantioselective HPLC determination and pharmacokinetic study of secnidazole enantiomers in rats.J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Aug 15;965:224-30. doi: 10.1016/j.jchromb.2014.06.036. Epub 2014 Jul 5. J Chromatogr B Analyt Technol Biomed Life Sci. 2014. PMID: 25049211
-
A specific chiral HPLC method for lifitegrast and determination of enantiomeric impurity in drug substance, ophthalmic product and stressed samples.J Pharm Biomed Anal. 2024 May 15;242:116039. doi: 10.1016/j.jpba.2024.116039. Epub 2024 Feb 15. J Pharm Biomed Anal. 2024. PMID: 38387128 Review.
-
Critical issues in chiral drug analysis in biological fluids by high-performance liquid chromatography.J Chromatogr B Biomed Appl. 1996 Nov 8;686(1):65-75. doi: 10.1016/s0378-4347(96)00274-5. J Chromatogr B Biomed Appl. 1996. PMID: 8953193 Review.
Cited by
-
Development and validation of Lenalidomide in human plasma by LC-MS/MS.Saudi J Biol Sci. 2019 Nov;26(7):1843-1847. doi: 10.1016/j.sjbs.2018.02.006. Epub 2018 Feb 13. Saudi J Biol Sci. 2019. PMID: 31762666 Free PMC article.
-
Quality by design based ecofriendly HPLC analytical method for simultaneous quantification of erastin and lenalidomide in mesoporous silica nanoparticles.Sci Rep. 2025 Mar 14;15(1):8873. doi: 10.1038/s41598-025-93331-8. Sci Rep. 2025. PMID: 40087405 Free PMC article.
References
-
- Kastritis E., Dimopoulos M.A.; The evolving role of lenalidomide in the treatment of hematologic malignancies; Expert Opinion on Pharmacotherapy, (2007); 8(4): 497–509. - PubMed
-
- Mitsiades C.S., Mitsiades N.; CC-5013 (Celgene); Current Opinion in Investigational Drugs, (2004); 5: 635–647. - PubMed
-
- List A., Kurtin S., Roe D.J., Buresh A., Mahadevan D., Fuchs D. et al. . Efficacy of lenalidomide in myelodysplastic syndromes; The New England Journal of Medicine, (2005); 352(6): 549–557. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

