Genetic and Dietary Regulation of Glyburide Efflux by the Human Placental Breast Cancer Resistance Protein Transporter
- PMID: 26850786
- PMCID: PMC4809313
- DOI: 10.1124/jpet.115.230185
Genetic and Dietary Regulation of Glyburide Efflux by the Human Placental Breast Cancer Resistance Protein Transporter
Abstract
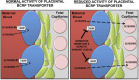
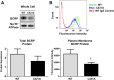
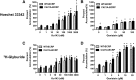
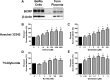
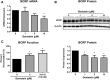
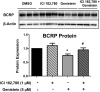
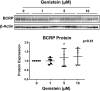
Glyburide is frequently used to treat gestational diabetes owing to its low fetal accumulation resulting from placental efflux by the breast cancer resistance protein (BCRP)/ABCG2 transporter. Here we sought to determine how exposure to the dietary phytoestrogen genistein and expression of a loss-of-function polymorphism in the ABCG2 gene (C421A) impacted the transport of glyburide by BCRP using stably transfected human embryonic kidney 293 (HEK) cells, human placental choriocarcinoma BeWo cells, and human placental explants. Genistein competitively inhibited the BCRP-mediated transport of (3)H-glyburide in both wild-type (WT) and C421A-BCRP HEK-expressing cells, with greater accumulation of (3)H-glyburide in cells expressing the C421A variant. In BeWo cells, exposure to genistein for 60 minutes increased the accumulation of (3)H-glyburide 30%-70% at concentrations relevant to dietary exposure (IC50 ∼180 nM). Continuous exposure of BeWo cells to genistein for 48 hours reduced the expression of BCRP mRNA and protein by up to 40%, which impaired BCRP transport activity. Pharmacologic antagonism of the estrogen receptor attenuated the genistein-mediated downregulation of BCRP expression, suggesting that phytoestrogens may reduce BCRP levels through this hormone receptor pathway in BeWo cells. Interestingly, genistein treatment for 48 hours did not alter BCRP protein expression in explants dissected from healthy term placentas. These data suggest that whereas genistein can act as a competitive inhibitor of BCRP-mediated transport, its ability to downregulate placental BCRP expression may only occur in choriocarcinoma cells. Overall, this research provides important mechanistic data regarding how the environment (dietary genistein) and a frequent genetic variant (ABCG2, C421A) may alter the maternal-fetal disposition of glyburide.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures

References
-
- Álvarez AI, Vallejo F, Barrera B, Merino G, Prieto JG, Tomás-Barberán F, Espín JC. (2011) Bioavailability of the glucuronide and sulfate conjugates of genistein and daidzein in breast cancer resistance protein 1 knockout mice. Drug Metab Dispos 39:2008–2012. - PubMed
-
- Anjalakshi C, Balaji V, Balaji MS, Seshiah V. (2007) A prospective study comparing insulin and glibenclamide in gestational diabetes mellitus in Asian Indian women. Diabetes Res Clin Pract 76:474–475. - PubMed
-
- Arai Y, Uehara M, Sato Y, Kimira M, Eboshida A, Adlercreutz H, Watanabe S. (2000) Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J Epidemiol 10:127–135. - PubMed
-
- Arias A, Rigalli JP, Villanueva SS, Ruiz ML, Luquita MG, Perdomo VG, Vore M, Catania VA, Mottino AD. (2014) Regulation of expression and activity of multidrug resistance proteins MRP2 and MDR1 by estrogenic compounds in Caco-2 cells: role in prevention of xenobiotic-induced cytotoxicity. Toxicology 320:46–55. - PubMed
-
- Atkinson DE, Sibley CP, Fairbairn LJ, Greenwood SL. (2006) MDR1 P-gp expression and activity in intact human placental tissue; upregulation by retroviral transduction. Placenta 27:707–714. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

