Spectrum of excess mortality due to carbapenem-resistant Klebsiella pneumoniae infections
- PMID: 26850824
- PMCID: PMC4930369
- DOI: 10.1016/j.cmi.2016.01.023
Spectrum of excess mortality due to carbapenem-resistant Klebsiella pneumoniae infections
Abstract
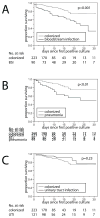
Patients infected or colonized with carbapenem-resistant Klebsiella pneumoniae (CRKp) are often chronically and acutely ill, which results in substantial mortality unrelated to infection. Therefore, estimating excess mortality due to CRKp infections is challenging. The Consortium on Resistance against Carbapenems in K. pneumoniae (CRACKLE) is a prospective multicenter study. Here, patients in CRACKLE were evaluated at the time of their first CRKp bloodstream infection (BSI), pneumonia or urinary tract infection (UTI). A control cohort of patients with CRKp urinary colonization without CRKp infection was constructed. Excess hospital mortality was defined as mortality in cases after subtracting mortality in controls. In addition, the adjusted hazard ratios (aHR) for time-to-hospital-mortality at 30 days associated with infection compared with colonization were calculated in Cox proportional hazard models. In the study period, 260 patients with CRKp infections were included in the BSI (90 patients), pneumonia (49 patients) and UTI (121 patients) groups, who were compared with 223 controls. All-cause hospital mortality in controls was 12%. Excess hospital mortality was 27% in both patients with BSI and those with pneumonia. Excess hospital mortality was not observed in patients with UTI. In multivariable analyses, BSI and pneumonia compared with controls were associated with aHR of 2.59 (95% CI 1.52-4.50, p <0.001) and 3.44 (95% CI 1.80-6.48, p <0.001), respectively. In conclusion, in patients with CRKp infection, pneumonia is associated with the highest excess hospital mortality. Patients with BSI have slightly lower excess hospital mortality rates, whereas excess hospital mortality was not observed in hospitalized patients with UTI.
Keywords: Klebsiella pneumoniae; carbapenem-resistant Enterobacteriaceae; epidemiology; mortality; pneumonia.
Copyright © 2016 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
All other authors: no conflicts reported
Figures
References
-
- Martin ET, Tansek R, Collins V, et al. The carbapenem-resistant enterobacteriaceae score: A bedside score to rule out infection with carbapenem-resistant enterobacteriaceae among hospitalized patients. Am J Infect Control. 2013;41:180–2. - PubMed
-
- Nguyen M, Eschenauer GA, Bryan M, et al. Carbapenem-resistant klebsiella pneumoniae bacteremia: Factors correlated with clinical and microbiologic outcomes. Diagn Microbiol Infect Dis. 67:180–4. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

