Cellular and molecular insight into the inhibition of primary root growth of Arabidopsis induced by peptaibols, a class of linear peptide antibiotics mainly produced by Trichoderma spp
- PMID: 26850879
- PMCID: PMC4809282
- DOI: 10.1093/jxb/erw023
Cellular and molecular insight into the inhibition of primary root growth of Arabidopsis induced by peptaibols, a class of linear peptide antibiotics mainly produced by Trichoderma spp
Abstract
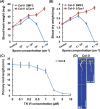
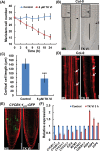
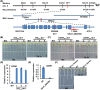
Trichoderma spp. are well known biocontrol agents that produce a variety of antibiotics. Peptaibols are a class of linear peptide antibiotics mainly produced by Trichoderma Alamethicin, the most studied peptaibol, is reported as toxic to plants at certain concentrations, while the mechanisms involved are unclear. We illustrated the toxic mechanisms of peptaibols by studying the growth-inhibitory effect of Trichokonin VI (TK VI), a peptaibol from Trichoderma longibrachiatum SMF2, on Arabidopsis primary roots. TK VI inhibited root growth by suppressing cell division and cell elongation, and disrupting root stem cell niche maintenance. TK VI increased auxin content and disrupted auxin response gradients in root tips. Further, we screened the Arabidopsis TK VI-resistant mutant tkr1. tkr1 harbors a point mutation in GORK, which encodes gated outwardly rectifying K(+)channel proteins. This mutation alleviated TK VI-induced suppression of K(+)efflux in roots, thereby stabilizing the auxin gradient. The tkr1 mutant also resisted the phytotoxicity of alamethicin. Our results indicate that GORK channels play a key role in peptaibol-plant interaction and that there is an inter-relationship between GORK channels and maintenance of auxin homeostasis. The cellular and molecular insight into the peptaibol-induced inhibition of plant root growth advances our understanding of Trichoderma-plant interactions.
Keywords: Arabidopsis thaliana; GORK; Trichoderma; Trichokonin VI.; auxin; peptaibols.
© The Author 2016. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures






Similar articles
-
The antibiotic peptaibol alamethicin from Trichoderma permeabilises Arabidopsis root apical meristem and epidermis but is antagonised by cellulase-induced resistance to alamethicin.BMC Plant Biol. 2018 Aug 10;18(1):165. doi: 10.1186/s12870-018-1370-x. BMC Plant Biol. 2018. PMID: 30097019 Free PMC article.
-
The volatile 6-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ETHYLENE INSENSITIVE 2 functioning.New Phytol. 2016 Mar;209(4):1496-512. doi: 10.1111/nph.13725. Epub 2015 Nov 16. New Phytol. 2016. PMID: 26568541
-
Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens.Microbiology (Reading). 2012 Jan;158(Pt 1):166-175. doi: 10.1099/mic.0.052670-0. Epub 2011 Nov 3. Microbiology (Reading). 2012. PMID: 22053006
-
Long-term live-cell imaging approaches to study lateral root formation in Arabidopsis thaliana.Microscopy (Oxf). 2019 Feb 1;68(1):4-12. doi: 10.1093/jmicro/dfy135. Microscopy (Oxf). 2019. PMID: 30476201 Review.
-
Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: interactions with plants.FEMS Microbiol Ecol. 2016 Apr;92(4):fiw036. doi: 10.1093/femsec/fiw036. Epub 2016 Feb 22. FEMS Microbiol Ecol. 2016. PMID: 26906097 Review.
Cited by
-
Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth.Int J Mol Sci. 2022 Feb 19;23(4):2329. doi: 10.3390/ijms23042329. Int J Mol Sci. 2022. PMID: 35216444 Free PMC article. Review.
-
Chromatographic and mass spectroscopic guided discovery of Trichoderma peptaibiotics and their bioactivity.Eng Microbiol. 2023 Dec 16;4(2):100135. doi: 10.1016/j.engmic.2023.100135. eCollection 2024 Jun. Eng Microbiol. 2023. PMID: 39629330 Free PMC article. Review.
-
The antibiotic peptaibol alamethicin from Trichoderma permeabilises Arabidopsis root apical meristem and epidermis but is antagonised by cellulase-induced resistance to alamethicin.BMC Plant Biol. 2018 Aug 10;18(1):165. doi: 10.1186/s12870-018-1370-x. BMC Plant Biol. 2018. PMID: 30097019 Free PMC article.
-
Environmental Growth Conditions of Trichoderma spp. Affects Indole Acetic Acid Derivatives, Volatile Organic Compounds, and Plant Growth Promotion.Front Plant Sci. 2017 Feb 9;8:102. doi: 10.3389/fpls.2017.00102. eCollection 2017. Front Plant Sci. 2017. PMID: 28232840 Free PMC article.
-
Integrated Translatome and Proteome: Approach for Accurate Portraying of Widespread Multifunctional Aspects of Trichoderma.Front Microbiol. 2017 Aug 29;8:1602. doi: 10.3389/fmicb.2017.01602. eCollection 2017. Front Microbiol. 2017. PMID: 28900417 Free PMC article. Review.
References
-
- Ache P, Becker D, Ivashikina N, Dietrich P, Roelfsema MRG, Hedrich R. 2000. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Letters 486, 93–98. - PubMed
-
- Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B. 2004. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119, 109–120. - PubMed
-
- Bechtold N, Pelletier G. 1998. In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods in Molecular Biology 82, 259–266. - PubMed
-
- Benítez T, Rincón AM, Limón MC, Codón AC. 2004. Biocontrol mechanisms of Trichoderma strains. International Microbiology 7, 249–260. - PubMed
-
- Béven L, Helluin O, Molle G, Duclohier H, Wróblewski H. 1999. Correlation between anti-bacterial activity and pore-sizes of two classes of voltage-dependent channel-forming peptides. Biochimica et Biophysica Acta 1420, 53–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

