Matrix metalloproteinase-9 deletion rescues auditory evoked potential habituation deficit in a mouse model of Fragile X Syndrome
- PMID: 26850918
- PMCID: PMC4785038
- DOI: 10.1016/j.nbd.2016.02.002
Matrix metalloproteinase-9 deletion rescues auditory evoked potential habituation deficit in a mouse model of Fragile X Syndrome
Abstract
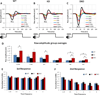
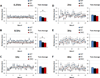
Sensory processing deficits are common in autism spectrum disorders, but the underlying mechanisms are unclear. Fragile X Syndrome (FXS) is a leading genetic cause of intellectual disability and autism. Electrophysiological responses in humans with FXS show reduced habituation with sound repetition and this deficit may underlie auditory hypersensitivity in FXS. Our previous study in Fmr1 knockout (KO) mice revealed an unusually long state of increased sound-driven excitability in auditory cortical neurons suggesting that cortical responses to repeated sounds may exhibit abnormal habituation as in humans with FXS. Here, we tested this prediction by comparing cortical event related potentials (ERP) recorded from wildtype (WT) and Fmr1 KO mice. We report a repetition-rate dependent reduction in habituation of N1 amplitude in Fmr1 KO mice and show that matrix metalloproteinase-9 (MMP-9), one of the known FMRP targets, contributes to the reduced ERP habituation. Our studies demonstrate a significant up-regulation of MMP-9 levels in the auditory cortex of adult Fmr1 KO mice, whereas a genetic deletion of Mmp-9 reverses ERP habituation deficits in Fmr1 KO mice. Although the N1 amplitude of Mmp-9/Fmr1 DKO recordings was larger than WT and KO recordings, the habituation of ERPs in Mmp-9/Fmr1 DKO mice is similar to WT mice implicating MMP-9 as a potential target for reversing sensory processing deficits in FXS. Together these data establish ERP habituation as a translation relevant, physiological pre-clinical marker of auditory processing deficits in FXS and suggest that abnormal MMP-9 regulation is a mechanism underlying auditory hypersensitivity in FXS.
Significance: Fragile X Syndrome (FXS) is the leading known genetic cause of autism spectrum disorders. Individuals with FXS show symptoms of auditory hypersensitivity. These symptoms may arise due to sustained neural responses to repeated sounds, but the underlying mechanisms remain unclear. For the first time, this study shows deficits in habituation of neural responses to repeated sounds in the Fmr1 KO mice as seen in humans with FXS. We also report an abnormally high level of matrix metalloprotease-9 (MMP-9) in the auditory cortex of Fmr1 KO mice and that deletion of Mmp-9 from Fmr1 KO mice reverses habituation deficits. These data provide a translation relevant electrophysiological biomarker for sensory deficits in FXS and implicate MMP-9 as a target for drug discovery.
Keywords: Auditory cortex; Autism; Cortical event related potentials; Fragile X Syndrome; Matrix metalloproteinase-9; Sensory hypersensitivity.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
We report no conflict of interest.
Figures




References
-
- Bailey DB, Jr, Mesibov GB, Hatton DD, Clark RD, Roberts JE, Mayhew L. Autistic behavior in young boys with fragile X syndrome. J Autism Dev Disord. 1998;28:499–508. - PubMed
-
- Bilousova TV, Dansie L, Ngo M, Aye J, Charles JR, Ethell DW, Ethell IM. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46(2):94–102. - PubMed
-
- Castren M, Paakkonen A, Tarkka IM, Ryynanen M, Partanen J. Augmentation of auditory N1 in children with fragile X syndrome. Brain Topography. 2003;15:165–171. - PubMed
-
- Chen L, Toth M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience. 2001;103:1043–1050. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

