Vestiges of Ent3p/Ent5p function in the giardial epsin homolog
- PMID: 26851076
- PMCID: PMC4775278
- DOI: 10.1016/j.bbamcr.2016.02.001
Vestiges of Ent3p/Ent5p function in the giardial epsin homolog
Abstract
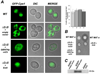
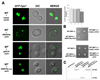
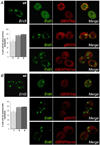
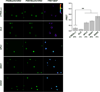
An accurate way to characterize the functional potential of a protein is to analyze recognized protein domains encoded by the genes in a given group. The epsin N-terminal homology (ENTH) domain is an evolutionarily conserved protein module found primarily in proteins that participate in clathrin-mediated trafficking. In this work, we investigate the function of the single ENTH-containing protein from the protist Giardia lamblia by testing its function in Saccharomyces cerevisiae. This protein, named GlENTHp (for G. lamblia ENTH protein), is involved in Giardia in endocytosis and in protein trafficking from the ER to the vacuoles, fulfilling the function of the ENTH proteins epsin and epsinR, respectively. There are two orthologs of epsin, Ent1p and Ent2p, and two orthologs of epsinR, Ent3p and Ent5p in S. cerevisiae. Although the expression of GlENTHp neither complemented growth in the ent1Δent2Δ mutant nor restored the GFP-Cps1 vacuolar trafficking defect in ent3Δent5Δ, it interfered with the normal function of Ent3/5 in the wild-type strain. The phenotype observed is linked to a defect in Cps1 localization and α-factor mating pheromone maturation. The finding that GlENTHp acts as dominant negative epsinR in yeast cells reinforces the phylogenetic data showing that GlENTHp belongs to the epsinR subfamily present in eukaryotes prior to their evolution into different taxa.
Keywords: ENTH motif; Endocytosis; Giardia lamblia; Vacuole; Vesicle transport; Yeast.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures




Similar articles
-
The giardial ENTH protein participates in lysosomal protein trafficking and endocytosis.Biochim Biophys Acta. 2015 Mar;1853(3):646-59. doi: 10.1016/j.bbamcr.2014.12.034. Epub 2015 Jan 6. Biochim Biophys Acta. 2015. PMID: 25576518
-
Dissecting Ent3p: the ENTH domain binds different SNAREs via distinct amino acid residues while the C-terminus is sufficient for retrograde transport from endosomes.Biochem J. 2010 Oct 1;431(1):123-34. doi: 10.1042/BJ20100693. Biochem J. 2010. PMID: 20658963
-
Distinct roles for TGN/endosome epsin-like adaptors Ent3p and Ent5p.Mol Biol Cell. 2006 Sep;17(9):3907-20. doi: 10.1091/mbc.e06-05-0410. Epub 2006 Jun 21. Mol Biol Cell. 2006. PMID: 16790491 Free PMC article.
-
ENTH/ANTH proteins and clathrin-mediated membrane budding.J Cell Sci. 2004 Jan 1;117(Pt 1):9-18. doi: 10.1242/jcs.00928. J Cell Sci. 2004. PMID: 14657269 Review.
-
Epsin: inducing membrane curvature.Int J Biochem Cell Biol. 2007;39(10):1765-70. doi: 10.1016/j.biocel.2006.12.004. Epub 2007 Jan 17. Int J Biochem Cell Biol. 2007. PMID: 17276129 Review.
References
-
- Traub LM. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim Biophys Acta. 2005;1744:415–437. - PubMed
-
- Owen DJ, Collins BM, Evans PR. Adaptors for clathrin coats: structure and function. Annu Rev Cell Dev Biol. 2004;20:153–191. - PubMed
-
- Itoh T, De Camilli P. BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochim Biophys Acta. 2006;1761:897–912. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

