Evaluating HPV-negative CIN2+ in the ATHENA trial
- PMID: 26851121
- PMCID: PMC5069615
- DOI: 10.1002/ijc.30032
Evaluating HPV-negative CIN2+ in the ATHENA trial
Abstract
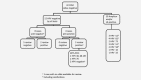
A post hoc analysis of the ATHENA study was performed to determine whether true HPV-negative cervical lesions occur and whether they have clinical relevance. The ATHENA database was searched for all CIN2 or worse (CIN2+) cases with cobas HPV-negative results and comparison was made with Linear Array (LA) and Amplicor to detect true false-negative HPV results. Immunostaining with p16 was performed on these cases to identify false-positive histology results. H&E slides were re-reviewed by the study pathologists with knowledge of patient age, HPV test results and p16 immunostaining. Those with positive p16 immunostaining and/or a positive histopathology review underwent whole tissue section HPV PCR by the SPF10/LiPA/RHA system. Among 46,887 eligible women, 497 cases of CIN2+ were detected, 55 of which tested negative by the cobas(®) HPV Test (32 CIN2, 23 CIN3/ACIS). By LA and/or Amplicor, 32 CIN2+ (20 CIN2, 12 CIN3/ACIS) were HPV positive and categorized as false-negatives by cobas HPV; nine of 12 false-negative CIN3/ACIS cases were p16+. There were 23 cases (12 CIN2, 11 CIN3/ACIS) negative by all HPV tests; seven of 11 CIN3/ACIS cases were p16+. H&E slides were available for six cases for re-review and all were confirmed as CIN3/ACIS. Tissue PCR was performed on the six confirmed CIN3/ACIS cases (and one without confirmation): four were positive for HPV types not considered oncogenic, two were positive for oncogenic genotypes and one was indeterminate. In summary, subanalysis of a large cervical cancer screening study did not identify any true CIN3/ACIS not attributable to HPV.
Keywords: HPV DNA testing; HPV genotype; adenocarcinoma in situ; cervical cancer screening; cervical intraepithelial neoplasia; histology.
© 2016 The Authors International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Figures
References
-
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12–19. - PubMed
-
- Liebrich C, Brummer O, Von Wasielewski R, et al. Primary cervical cancer truly negative for high‐risk human papillomavirus is a rare but distinct entity that can affect virgins and young adolescents. Eur J Gynaecol Oncol 2009;30:45–8. - PubMed
-
- Böhmer G, van den Brule AJ, Brummer O, et al. No confirmed case of human papillomavirus DNA‐negative cervical intraepithelial neoplasia grade 3 or invasive primary cancer of the uterine cervix among 511 patients. Am J Obstet Gynecol 2003;189:118–20. - PubMed
-
- Kurman RJ, Carcangiu ML, Herrington CS, et al., eds. WHO classification of tumors of female reproductive organs, 4th edn., vol. 6 Geneva: World Health Organization, 2014.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical