Implantation of healthy matrix-embedded endothelial cells rescues dysfunctional endothelium and ischaemic tissue in liver engraftment
- PMID: 26851165
- PMCID: PMC5288307
- DOI: 10.1136/gutjnl-2015-310409
Implantation of healthy matrix-embedded endothelial cells rescues dysfunctional endothelium and ischaemic tissue in liver engraftment
Abstract
Objective: Liver transplantation is limited by ischaemic injury which promotes endothelial cell and hepatocyte dysfunction and eventually organ failure. We sought to understand how endothelial state determines liver recovery after hepatectomy and engraftment.
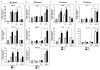
Design: Matrix-embedded endothelial cells (MEECs) with retained healthy phenotype or control acellular matrices were implanted in direct contact with the remaining median lobe of donor mice undergoing partial hepatectomy (70%), or in the interface between the remaining median lobe and an autograft or isograft from the left lobe in hepatectomised recipient mice. Hepatic vascular architecture, DNA fragmentation and apoptosis in the median lobe and grafts, serum markers of liver damage and phenotype of macrophage and lymphocyte subsets in the liver after engraftment were analysed 7 days post-op.
Results: Healthy MEECs create a functional vascular splice in donor and recipient liver after 70% hepatectomy in mouse protecting these livers from ischaemic injury, hepatic congestion and inflammation. Macrophages recruited adjacent to the vascular nodes into the implants switched to an anti-inflammatory and regenerative profile M2. MEECs improved liver function and the rate of liver regeneration and prevented apoptosis in donor liver lobes, autologous grafts and syngeneic engraftment.
Conclusions: Implants with healthy endothelial cells rescue liver donor and recipient endothelium and parenchyma from ischaemic injury after major hepatectomy and engraftment. This study highlights endothelial-hepatocyte crosstalk in hepatic repair and provides a promising new approach to improve regenerative medicine outcomes and liver transplantation.
Keywords: ENDOTHELIAL CELLS; ISCHAEMIA; LIVER IMMUNOLOGY; LIVER REGENERATION.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

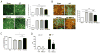


References
-
- Lim YS, Kim WR. The global impact of hepatic fibrosis and end-stage liver disease. Clin Liver Dis. 2008;12:733–746. - PubMed
-
- US Department of Health and Human Services. Organ Procurement and Transplantation Network. 2014 [online], http://optn.transplant.hrsa.gov/data/
-
- Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
