Regulation of myofibroblast differentiation by cardiac glycosides
- PMID: 26851261
- PMCID: PMC4867347
- DOI: 10.1152/ajplung.00322.2015
Regulation of myofibroblast differentiation by cardiac glycosides
Abstract
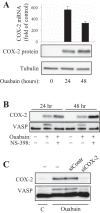
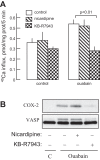
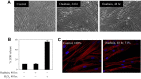
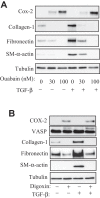
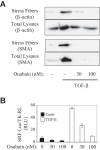
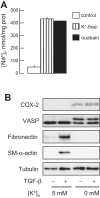
Myofibroblast differentiation is a key process in pathogenesis of fibrotic diseases. Cardiac glycosides (ouabain, digoxin) inhibit Na(+)-K(+)-ATPase, resulting in increased intracellular [Na(+)]-to-[K(+)] ratio in cells. Microarray analysis suggested that increased intracellular [Na(+)]/[K(+)] ratio may promote the expression of cyclooxygenase-2 (COX-2), a critical enzyme in the synthesis of prostaglandins. Given antifibrotic effects of prostaglandins through activation of protein kinase A (PKA), we examined if cardiac glycosides stimulate COX-2 expression in human lung fibroblasts and how they affect myofibroblast differentiation. Ouabain stimulated a profound COX-2 expression and a sustained PKA activation, which was blocked by COX-2 inhibitor or by COX-2 knockdown. Ouabain-induced COX-2 expression and PKA activation were abolished by the inhibitor of the Na(+)/Ca(2+) exchanger, KB-R4943. Ouabain inhibited transforming growth factor-β (TGF-β)-induced Rho activation, stress fiber formation, serum response factor activation, and the expression of smooth muscle α-actin, collagen-1, and fibronectin. These effects were recapitulated by an increase in intracellular [Na(+)]/[K(+)] ratio through the treatment of cells with K(+)-free medium or with digoxin. Although inhibition of COX-2 or of the Na(+)/Ca(2+) exchanger blocked ouabain-induced PKA activation, this failed to reverse the inhibition of TGF-β-induced Rho activation or myofibroblast differentiation by ouabain. Together, these data demonstrate that ouabain, through the increase in intracellular [Na(+)]/[K(+)] ratio, drives the induction of COX-2 expression and PKA activation, which is accompanied by a decreased Rho activation and myofibroblast differentiation in response to TGF-β. However, COX-2 expression and PKA activation are not sufficient for inhibition of the fibrotic effects of TGF-β by ouabain, suggesting that additional mechanisms must exist.
Keywords: cyclooxygenase-2; digoxin; intracellular sodium-to-potassium ion ratio; ouabain.
Copyright © 2016 the American Physiological Society.
Figures


Similar articles
-
Na+,K+-ATPase as a Target for Treatment of Tissue Fibrosis.Curr Med Chem. 2019;26(3):564-575. doi: 10.2174/0929867324666170619105407. Curr Med Chem. 2019. PMID: 28625151 Review.
-
Regulation of myofibroblast differentiation and bleomycin-induced pulmonary fibrosis by adrenomedullin.Am J Physiol Lung Cell Mol Physiol. 2013 Jun 1;304(11):L757-64. doi: 10.1152/ajplung.00262.2012. Epub 2013 Apr 12. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23585227 Free PMC article.
-
Repurposed drug screen identifies cardiac glycosides as inhibitors of TGF-β-induced cancer-associated fibroblast differentiation.Oncotarget. 2016 May 31;7(22):32200-9. doi: 10.18632/oncotarget.8609. Oncotarget. 2016. PMID: 27058757 Free PMC article.
-
Cardiac glycosides induced toxicity in human cells expressing α1-, α2-, or α3-isoforms of Na-K-ATPase.Am J Physiol Cell Physiol. 2015 Jul 15;309(2):C126-35. doi: 10.1152/ajpcell.00089.2015. Am J Physiol Cell Physiol. 2015. PMID: 25994790
-
Control of lung myofibroblast transformation by monovalent ion transporters.Curr Top Membr. 2019;83:15-43. doi: 10.1016/bs.ctm.2019.01.002. Epub 2019 Feb 14. Curr Top Membr. 2019. PMID: 31196603 Review.
Cited by
-
Novel high-throughput myofibroblast assays identify agonists with therapeutic potential in pulmonary fibrosis that act via EP2 and EP4 receptors.PLoS One. 2018 Nov 28;13(11):e0207872. doi: 10.1371/journal.pone.0207872. eCollection 2018. PLoS One. 2018. PMID: 30485339 Free PMC article.
-
Ouabain ameliorates bleomycin induced pulmonary fibrosis by inhibiting proliferation and promoting apoptosis of lung fibroblasts.Am J Transl Res. 2018 Sep 15;10(9):2967-2974. eCollection 2018. Am J Transl Res. 2018. PMID: 30323883 Free PMC article.
-
Proinflammatory Effects of Cardiotonic Steroids Mediated by NKA α-1 (Na+/K+-ATPase α-1)/Src Complex in Renal Epithelial Cells and Immune Cells.Hypertension. 2019 Jul;74(1):73-82. doi: 10.1161/HYPERTENSIONAHA.118.12605. Epub 2019 May 28. Hypertension. 2019. PMID: 31132948 Free PMC article.
-
Cardiotonic Steroids and the Sodium Trade Balance: New Insights into Trade-Off Mechanisms Mediated by the Na⁺/K⁺-ATPase.Int J Mol Sci. 2018 Aug 30;19(9):2576. doi: 10.3390/ijms19092576. Int J Mol Sci. 2018. PMID: 30200235 Free PMC article. Review.
-
Hypoxic Stress-Dependent Regulation of Na,K-ATPase in Ischemic Heart Disease.Int J Mol Sci. 2023 Apr 26;24(9):7855. doi: 10.3390/ijms24097855. Int J Mol Sci. 2023. PMID: 37175562 Free PMC article. Review.
References
-
- Abramochkin DV, Vornanen M. Inhibition of the cardiac ATP-dependent potassium current by KB-R7943. Comp Biochem Physiol A Mol Integr Physiol 175: 38–45, 2014. - PubMed
-
- Akimova OA, Bagrov AY, Lopina OD, Kamernitsky AV, Tremblay J, Hamet P, Orlov SN. Cardiotonic steroids differentially affect intracellular Na+ and [Na+]i/[K+]i-independent signaling in C7-MDCK cells. J Biol Chem 280: 832–839, 2005. - PubMed
-
- Aperia A. New roles for an old enzyme: Na,K-ATPase emerges as an interesting drug target. J Intern Med 261: 44–52, 2007. - PubMed
-
- Billman GE. KB-R7943. Kanebo. Curr Opin Investig Drugs 2: 1740–1745, 2001. - PubMed
-
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev 79: 763–854, 1999. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

