Modulation of Intrinsically Disordered Protein Function by Post-translational Modifications
- PMID: 26851279
- PMCID: PMC4807257
- DOI: 10.1074/jbc.R115.695056
Modulation of Intrinsically Disordered Protein Function by Post-translational Modifications
Abstract
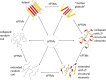
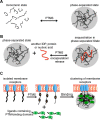
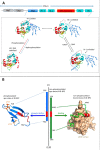
Post-translational modifications (PTMs) produce significant changes in the structural properties of intrinsically disordered proteins (IDPs) by affecting their energy landscapes. PTMs can induce a range of effects, from local stabilization or destabilization of transient secondary structure to global disorder-to-order transitions, potentially driving complete state changes between intrinsically disordered and folded states or dispersed monomeric and phase-separated states. Here, we discuss diverse biological processes that are dependent on PTM regulation of IDPs. We also present recent tools for generating homogenously modified IDPs for studies of PTM-mediated IDP regulatory mechanisms.
Keywords: intrinsically disordered protein; post-translational modification (PTM); protein conformation; protein-DNA interaction; protein-protein interaction; regulation.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Dyson H. J., and Wright P. E. (2005) Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 6, 197–208 - PubMed
-
- van der Lee R., Buljan M., Lang B., Weatheritt R. J., Daughdrill G. W., Dunker A. K., Fuxreiter M., Gough J., Gsponer J., Jones D. T., Kim P. M., Kriwacki R. W., Oldfield C. J., Pappu R. V., Tompa P., Uversky V. N., Wright P. E., and Babu M. M. (2014) Classification of intrinsically disordered regions and proteins. Chem. Rev. 114, 6589–6631 - PMC - PubMed
-
- Xie H., Vucetic S., Iakoucheva L. M., Oldfield C. J., Dunker A. K., Obradovic Z., and Uversky V. N. (2007) Functional anthology of intrinsic disorder. 3. Ligands, post-translational modifications, and diseases associated with intrinsically disordered proteins. J. Proteome Res. 6, 1917–1932 - PMC - PubMed
-
- Dosztányi Z., Chen J., Dunker A. K., Simon I., and Tompa P. (2006) Disorder and sequence repeats in hub proteins and their implications for network evolution. J. Proteome Res. 5, 2985–2995 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

