Dancing Protein Clouds: The Strange Biology and Chaotic Physics of Intrinsically Disordered Proteins
- PMID: 26851286
- PMCID: PMC4807255
- DOI: 10.1074/jbc.R115.685859
Dancing Protein Clouds: The Strange Biology and Chaotic Physics of Intrinsically Disordered Proteins
Abstract
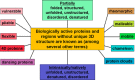
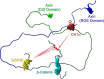
Biologically active but floppy proteins represent a new reality of modern protein science. These intrinsically disordered proteins (IDPs) and hybrid proteins containing ordered and intrinsically disordered protein regions (IDPRs) constitute a noticeable part of any given proteome. Functionally, they complement ordered proteins, and their conformational flexibility and structural plasticity allow them to perform impossible tricks and be engaged in biological activities that are inaccessible to well folded proteins with their unique structures. The major goals of this minireview are to show that, despite their simplified amino acid sequences, IDPs/IDPRs are complex entities often resembling chaotic systems, are structurally and functionally heterogeneous, and can be considered an important part of the structure-function continuum. Furthermore, IDPs/IDPRs are everywhere, and are ubiquitously engaged in various interactions characterized by a wide spectrum of binding scenarios and an even wider spectrum of structural and functional outputs.
Keywords: complexity; dynamical system; intrinsically disordered protein; intrinsically disordered proteins; multifunctional protein; post-translational modification (PTM); posttranslational modification; protein folding; protein-protein interaction; structural heterogeneity.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Modulation of Intrinsically Disordered Protein Function by Post-translational Modifications.J Biol Chem. 2016 Mar 25;291(13):6696-705. doi: 10.1074/jbc.R115.695056. Epub 2016 Feb 5. J Biol Chem. 2016. PMID: 26851279 Free PMC article. Review.
-
Functions of short lifetime biological structures at large: the case of intrinsically disordered proteins.Brief Funct Genomics. 2020 Jan 22;19(1):60-68. doi: 10.1093/bfgp/ely023. Brief Funct Genomics. 2020. PMID: 29982297 Review.
-
Intrinsic Disorder, Protein-Protein Interactions, and Disease.Adv Protein Chem Struct Biol. 2018;110:85-121. doi: 10.1016/bs.apcsb.2017.06.005. Epub 2017 Jul 24. Adv Protein Chem Struct Biol. 2018. PMID: 29413001 Review.
-
Introduction to the Thematic Minireview Series on Intrinsically Disordered Proteins.J Biol Chem. 2016 Mar 25;291(13):6679-80. doi: 10.1074/jbc.R116.719930. Epub 2016 Feb 5. J Biol Chem. 2016. PMID: 26851284 Free PMC article.
-
Intrinsically disordered proteins in crowded milieu: when chaos prevails within the cellular gumbo.Cell Mol Life Sci. 2018 Nov;75(21):3907-3929. doi: 10.1007/s00018-018-2894-9. Epub 2018 Jul 31. Cell Mol Life Sci. 2018. PMID: 30066087 Free PMC article. Review.
Cited by
-
Paradoxes and wonders of intrinsic disorder: Stability of instability.Intrinsically Disord Proteins. 2017 Oct 16;5(1):e1327757. doi: 10.1080/21690707.2017.1327757. eCollection 2017. Intrinsically Disord Proteins. 2017. PMID: 30250771 Free PMC article.
-
Epichromatin and chromomeres: a 'fuzzy' perspective.Open Biol. 2018 Jun;8(6):180058. doi: 10.1098/rsob.180058. Open Biol. 2018. PMID: 29875200 Free PMC article. Review.
-
Intrinsic Disorder and Other Malleable Arsenals of Evolved Protein Multifunctionality.J Mol Evol. 2024 Dec;92(6):669-684. doi: 10.1007/s00239-024-10196-7. Epub 2024 Aug 30. J Mol Evol. 2024. PMID: 39214891 Review.
-
Bacterial functional amyloids: Order from disorder.Biochim Biophys Acta Proteins Proteom. 2019 Oct;1867(10):954-960. doi: 10.1016/j.bbapap.2019.05.010. Epub 2019 Jun 10. Biochim Biophys Acta Proteins Proteom. 2019. PMID: 31195143 Free PMC article. Review.
-
Exploring the functional impact of alternative splicing on human protein isoforms using available annotation sources.Brief Bioinform. 2019 Sep 27;20(5):1754-1768. doi: 10.1093/bib/bby047. Brief Bioinform. 2019. PMID: 29931155 Free PMC article. Review.
References
-
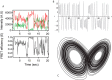
- Lorenz E. N. (1963) Deterministic nonperiodic flow. J. Atmos. Sci. 20, 130–141
-
- Lorenz E. N. (1963) The predictability of hydrodynamic flow. Trans. N. Y. Acad. Sci. 25, 409–432
-
- Stewart I. (2011) Sources of uncertainty in deterministic dynamics: an informal overview. Philos. Trans. A Math. Phys. Eng. Sci. 369, 4705–4729 - PubMed
-
- Dunker A. K., Garner E., Guilliot S., Romero P., Albrecht K., Hart J., Obradovic Z., Kissinger C., and Villafranca J. E. (1998) Protein disorder and the evolution of molecular recognition: theory, predictions and observations. Pac. Symp. Biocomput. 473–484 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

