Glial coverage in the optic nerve expands in proportion to optic axon loss in chronic mouse glaucoma
- PMID: 26851485
- PMCID: PMC4972706
- DOI: 10.1016/j.exer.2016.01.014
Glial coverage in the optic nerve expands in proportion to optic axon loss in chronic mouse glaucoma
Abstract
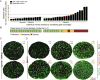
Within the white matter, axonal loss by neurodegeneration is coupled to glial cell changes in gene expression, structure and function commonly termed gliosis. Recently, we described the highly variable expansion of gliosis alebosco@neuro.utah.edu in degenerative optic nerves from the DBA/2J mouse model of chronic, age-related glaucoma. Here, to estimate and compare the levels of axonal loss with the expansion of glial coverage and axonal degeneration in DBA/2J nerves, we combined semiautomatic axon counts with threshold-based segmentation of total glial/scar areas and degenerative axonal profiles in plastic cross-sections. In nerves ranging from mild to severe degeneration, we found that the progression of axonal dropout is coupled to an increase of gliotic area. We detected a strong correlation between axon loss and the aggregate coverage by glial cells and scar, whereas axon loss did not correlate with the small fraction of degenerating profiles. Nerves with low to medium levels of axon loss displayed moderate glial reactivity, consisting of hypertrophic astrocytes, activated microglia and normal distribution of oligodendrocytes, with minimal reorganization of the tissue architecture. In contrast, nerves with extensive axonal loss showed prevalent rearrangement of the nerve, with loss of axon fascicle territories and enlarged or almost continuous gliotic and scar domains, containing reactive astrocytes, oligodendrocytes and activated microglia. These findings support the value of optic nerve gliotic expansion as a quantitative estimate of optic neuropathy that correlates with axon loss, applicable to grade the severity of optic nerve damage in mouse chronic glaucoma.
Keywords: Axon loss; Glaucoma; Gliosis; Microglia; Neurodegeneration; Optic nerve; Remodeling; Segmentation.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures




References
-
- Buckingham BP, Inman DM, Lambert W, Oglesby E, Calkins DJ, Steele MR, Vetter ML, Marsh-Armstrong N, Horner PJ. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:2735–2744. - PMC - PubMed
-
- Buffo A, Vosko MR, Erturk D, Hamann GF, Jucker M, Rowitch D, Gotz M. Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18183–18188. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

