Neuron class-specific requirements for Fragile X Mental Retardation Protein in critical period development of calcium signaling in learning and memory circuitry
- PMID: 26851502
- PMCID: PMC4785039
- DOI: 10.1016/j.nbd.2016.02.006
Neuron class-specific requirements for Fragile X Mental Retardation Protein in critical period development of calcium signaling in learning and memory circuitry
Abstract
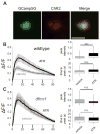
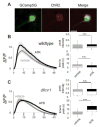
Neural circuit optimization occurs through sensory activity-dependent mechanisms that refine synaptic connectivity and information processing during early-use developmental critical periods. Fragile X Mental Retardation Protein (FMRP), the gene product lost in Fragile X syndrome (FXS), acts as an activity sensor during critical period development, both as an RNA-binding translation regulator and channel-binding excitability regulator. Here, we employ a Drosophila FXS disease model to assay calcium signaling dynamics with a targeted transgenic GCaMP reporter during critical period development of the mushroom body (MB) learning/memory circuit. We find FMRP regulates depolarization-induced calcium signaling in a neuron-specific manner within this circuit, suppressing activity-dependent calcium transients in excitatory cholinergic MB input projection neurons and enhancing calcium signals in inhibitory GABAergic MB output neurons. Both changes are restricted to the developmental critical period and rectified at maturity. Importantly, conditional genetic (dfmr1) rescue of null mutants during the critical period corrects calcium signaling defects in both neuron classes, indicating a temporally restricted FMRP requirement. Likewise, conditional dfmr1 knockdown (RNAi) during the critical period replicates constitutive null mutant defects in both neuron classes, confirming cell-autonomous requirements for FMRP in developmental regulation of calcium signaling dynamics. Optogenetic stimulation during the critical period enhances depolarization-induced calcium signaling in both neuron classes, but this developmental change is eliminated in dfmr1 null mutants, indicating the activity-dependent regulation requires FMRP. These results show FMRP shapes neuron class-specific calcium signaling in excitatory vs. inhibitory neurons in developing learning/memory circuitry, and that FMRP mediates activity-dependent regulation of calcium signaling specifically during the early-use critical period.
Keywords: Activity-dependent; Autism spectrum disorder; Drosophila; Excitation vs. inhibition; Fragile X syndrome; Optogenetics.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

