Harvesting the biosynthetic machineries that cultivate a variety of indispensable plant natural products
- PMID: 26851514
- PMCID: PMC5217535
- DOI: 10.1016/j.cbpa.2016.01.008
Harvesting the biosynthetic machineries that cultivate a variety of indispensable plant natural products
Abstract
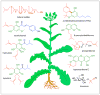
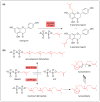
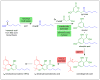
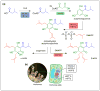
Plants are a sustainable resource for valuable natural chemicals best illustrated by large-scale farming centered on specific products. Here, we review recent discoveries of plant metabolic pathways producing natural products with unconventional biomolecular structures. Prenylation of polyketides by aromatic prenyltransferases (aPTases) ties together two of the major groups of plant specialized chemicals, terpenoids and polyketides, providing a core modification leading to new bioactivities and downstream metabolic processing. Moreover, PTases that biosynthesize Z-terpenoid precursors for small molecules such as lycosantalene have recently been found in the tomato family. Gaps in our understanding of how economically important compounds such as cannabinoids are produced are being identified using next-generation 'omics' to rapidly advance biochemical breakthroughs at an unprecedented rate. For instance, olivetolic acid cyclase, a polyketide synthase (PKS) co-factor from Cannabis sativa, directs the proper cyclization of a polyketide intermediate. Elucidations of spatial and temporal arrangements of biosynthetic enzymes into metabolons, such as those used to control the efficient production of natural polymers such as rubber and defensive small molecules such as linamarin and lotaustralin, provide blueprints for engineering streamlined production of plant products.
Copyright © 2016 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
Similar articles
-
Engineered Biosynthesis of Medicinally Important Plant Natural Products in Microorganisms.Curr Top Med Chem. 2016;16(15):1740-54. doi: 10.2174/1568026616666151012112637. Curr Top Med Chem. 2016. PMID: 26456465 Review.
-
Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides.Proc Natl Acad Sci U S A. 2012 Jul 31;109(31):12811-6. doi: 10.1073/pnas.1200330109. Epub 2012 Jul 16. Proc Natl Acad Sci U S A. 2012. PMID: 22802619 Free PMC article.
-
Another level of complex-ity: The role of metabolic channeling and metabolons in plant terpenoid metabolism.Front Plant Sci. 2022 Aug 10;13:954083. doi: 10.3389/fpls.2022.954083. eCollection 2022. Front Plant Sci. 2022. PMID: 36035727 Free PMC article. Review.
-
Structural basis for the promiscuous biosynthetic prenylation of aromatic natural products.Nature. 2005 Jun 16;435(7044):983-7. doi: 10.1038/nature03668. Nature. 2005. PMID: 15959519 Free PMC article.
-
Microbial production of small medicinal molecules and biologics: From nature to synthetic pathways.Biotechnol Adv. 2018 Dec;36(8):2219-2231. doi: 10.1016/j.biotechadv.2018.10.009. Epub 2018 Oct 29. Biotechnol Adv. 2018. PMID: 30385278 Review.
Cited by
-
A coupled in vitro/in vivo approach for engineering a heterologous type III PKS to enhance polyketide biosynthesis in Saccharomyces cerevisiae.Biotechnol Bioeng. 2018 Jun;115(6):1394-1402. doi: 10.1002/bit.26564. Epub 2018 Mar 13. Biotechnol Bioeng. 2018. PMID: 29457628 Free PMC article.
-
Biosynthetic potential of sesquiterpene synthases: product profiles of Egyptian Henbane premnaspirodiene synthase and related mutants.J Antibiot (Tokyo). 2016 Jul;69(7):524-33. doi: 10.1038/ja.2016.68. Epub 2016 Jun 22. J Antibiot (Tokyo). 2016. PMID: 27328867
-
Engineering of Metabolic Pathways Using Synthetic Enzyme Complexes.Plant Physiol. 2019 Mar;179(3):918-928. doi: 10.1104/pp.18.01280. Epub 2018 Nov 19. Plant Physiol. 2019. PMID: 30455287 Free PMC article. Review.
-
A Novel Class of Plant Type III Polyketide Synthase Involved in Orsellinic Acid Biosynthesis from Rhododendron dauricum.Front Plant Sci. 2016 Sep 27;7:1452. doi: 10.3389/fpls.2016.01452. eCollection 2016. Front Plant Sci. 2016. PMID: 27729920 Free PMC article.
-
Engineering Plant Secondary Metabolism in Microbial Systems.Plant Physiol. 2019 Mar;179(3):844-861. doi: 10.1104/pp.18.01291. Epub 2019 Jan 14. Plant Physiol. 2019. PMID: 30643013 Free PMC article.
References
-
- Ayseli MT, Ayseli Yİ. Flavors of the future: health benefits of flavor precursors and volatile compounds in plant foods. Trends Food Sci Technol. 2015 http://dx.doi.org/10.1016/j.tifs.2015.11.005. - DOI
-
- Lange BM. The evolution of plant secretory structures and emergence of terpenoid chemical diversity. Annu Rev Plant Biol. 2015;66:139–159. - PubMed
-
- Stewart C, Vickery CR, Burkart MD, Noel JP. Confluence of structural and chemical biology: plant polyketide synthases as biocatalysts for a bio-based future. Curr Opin Plant Biol. 2013;16:365–372. - PubMed
-
- Laursen T, Møller BL, Bassard J-E. Plasticity of specialized metabolism as mediated by dynamic metabolons. Trends Plant Sci. 2015;20:20–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

