Nano-biosensors to detect beta-amyloid for Alzheimer's disease management
- PMID: 26851586
- PMCID: PMC4786026
- DOI: 10.1016/j.bios.2016.01.065
Nano-biosensors to detect beta-amyloid for Alzheimer's disease management
Abstract
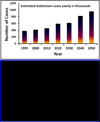
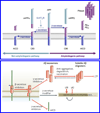
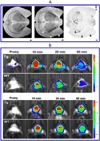
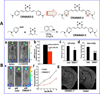
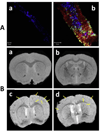
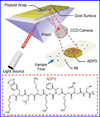
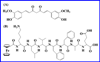
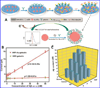
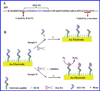
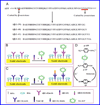
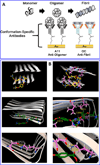
Beta-amyloid (β-A) peptides are potential biomarkers to monitor Alzheimer's diseases (AD) for diagnostic purposes. Increased β-A level is neurotoxic and induces oxidative stress in brain resulting in neurodegeneration and causes dementia. As of now, no sensitive and inexpensive method is available for β-A detection under physiological and pathological conditions. Although, available methods such as neuroimaging, enzyme-linked immunosorbent assay (ELISA), and polymerase chain reaction (PCR) detect β-A, but they are not yet extended at point-of-care (POC) due to sophisticated equipments, need of high expertize, complicated operations, and challenge of low detection limit. Recently, β-A antibody based electrochemical immuno-sensing approach has been explored to detect β-A at pM levels within 30-40 min compared to 6-8h of ELISA test. The introduction of nano-enabling electrochemical sensing technology could enable rapid detection of β-A at POC and may facilitate fast personalized health care delivery. This review explores recent advancements in nano-enabling electrochemical β-A sensing technologies towards POC application to AD management. These analytical tools can serve as an analytical tool for AD management program to obtain bio-informatics needed to optimize therapeutics for neurodegenerative diseases diagnosis management.
Keywords: Alzheimer's diseases; Beta-amyloid; Diseases management; Electrochemical sensor; Point-of-care sensing.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures










References
-
- Adle-Biassette H, Levy Y, Colornbel M, Poron F, Natchev S, Keohane C, Gray F. Neuropathol. Appl. Neurobiol. 1995;21(3):218–227. - PubMed
-
- Beduer A, Joris P, Mosser S, Fraering PC, Renaud P. J. Neural. Eng. 2015;12(2):024001. - PubMed
-
- Bogomolova A, Komarova E, Reber K, Gerasimov T, Yavuz O, Bhatt S, Aldissi M. Anal. Chem. 2009;81(10):3944–3949. - PubMed
-
- Butterfield DA, Drake J, Pocernich C, Castegna A. Trend Mol. Med. 2001;7(12):548–554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

