Frogs as integrative models for understanding digestive organ development and evolution
- PMID: 26851628
- PMCID: PMC4798877
- DOI: 10.1016/j.semcdb.2016.02.001
Frogs as integrative models for understanding digestive organ development and evolution
Abstract
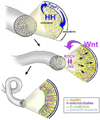
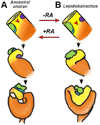
The digestive system comprises numerous cells, tissues and organs that are essential for the proper assimilation of nutrients and energy. Many aspects of digestive organ function are highly conserved among vertebrates, yet the final anatomical configuration of the gut varies widely between species, especially those with different diets. Improved understanding of the complex molecular and cellular events that orchestrate digestive organ development is pertinent to many areas of biology and medicine, including the regeneration or replacement of diseased organs, the etiology of digestive organ birth defects, and the evolution of specialized features of digestive anatomy. In this review, we highlight specific examples of how investigations using Xenopus laevis frog embryos have revealed insight into the molecular and cellular dynamics of digestive organ patterning and morphogenesis that would have been difficult to obtain in other animal models. Additionally, we discuss recent studies of gut development in non-model frog species with unique feeding strategies, such as Lepidobatrachus laevis and Eleutherodactylous coqui, which are beginning to provide glimpses of the evolutionary mechanisms that may generate morphological variation in the digestive tract. The unparalleled experimental versatility of frog embryos make them excellent, integrative models for studying digestive organ development across multiple disciplines.
Keywords: Eleutherodactylous coqui; Embryo; Evolution; Gut; Lepidobatrachus; Morphogenesis; Specification; Xenopus.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


References
-
- Keller R. Cell migration during gastrulation. Current opinion in cell biology. 2005;17:533–541. - PubMed
-
- Keller R, Davidson LA, Shook DR. How we are shaped: the biomechanics of gastrulation. Differentiation. 2003;71:171–205. - PubMed
-
- Spemann H, Mangold H. Induction of embryonic primordia by implantation of organizers from a different species. 1923. Int J Dev Biol. 2001;45:13–38. - PubMed
-
- Blitz IL, Andelfinger G, Horb ME. Germ layers to organs: using Xenopus to study “later” development. Seminars in cell & developmental biology. 2006;17:133–145. - PubMed
-
- Nieuwkoop PaF J. Normal Table of Xenopus Laevis (Daudin) New York, NY: Garland Publishing Inc; 1994.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

