Cancer-associated fibroblasts promote the progression of endometrial cancer via the SDF-1/CXCR4 axis
- PMID: 26851944
- PMCID: PMC4744391
- DOI: 10.1186/s13045-015-0231-4
Cancer-associated fibroblasts promote the progression of endometrial cancer via the SDF-1/CXCR4 axis
Abstract
Background: Cancer-associated fibroblasts (CAFs) are believed to play an essential role in cancer initiation and development. However, little research has been undertaken to evaluate the role of CAFs in endometrial cancer (EC) progression. We aim to detect the functional contributions of CAFs to promote progression of EC.
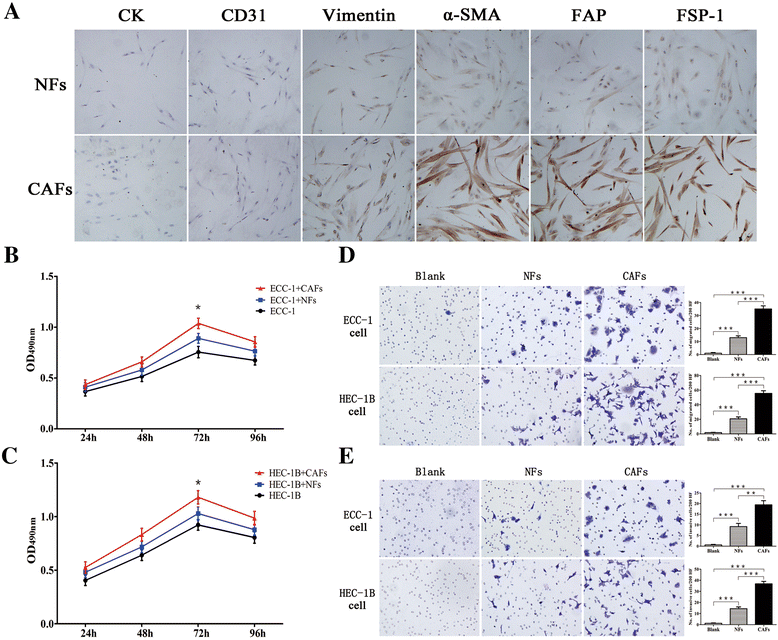
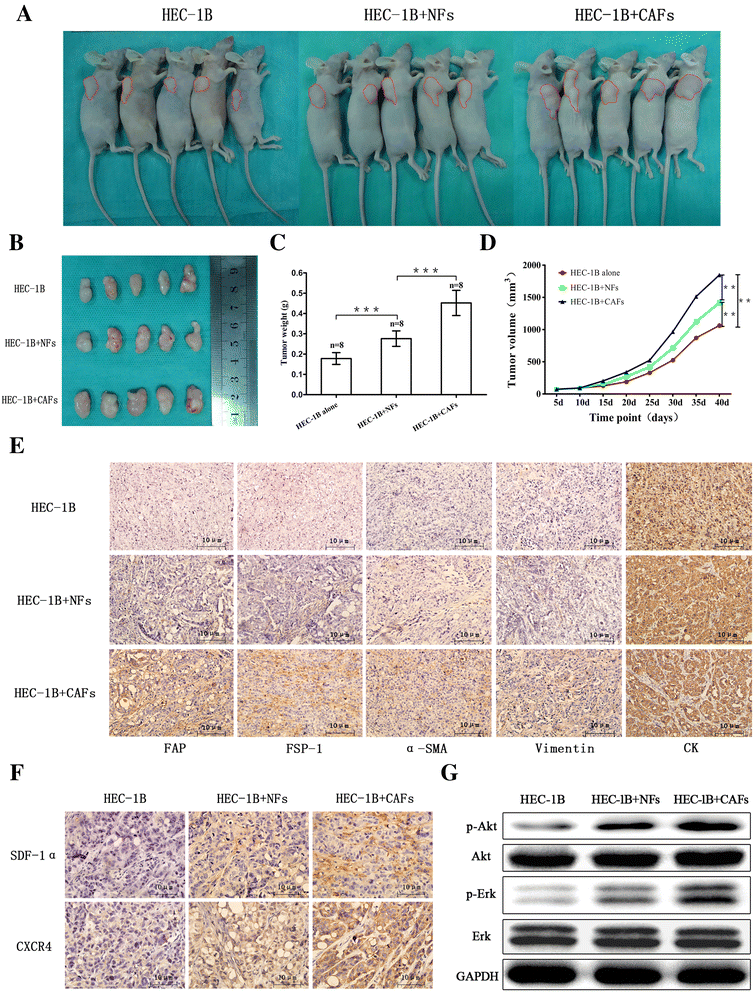
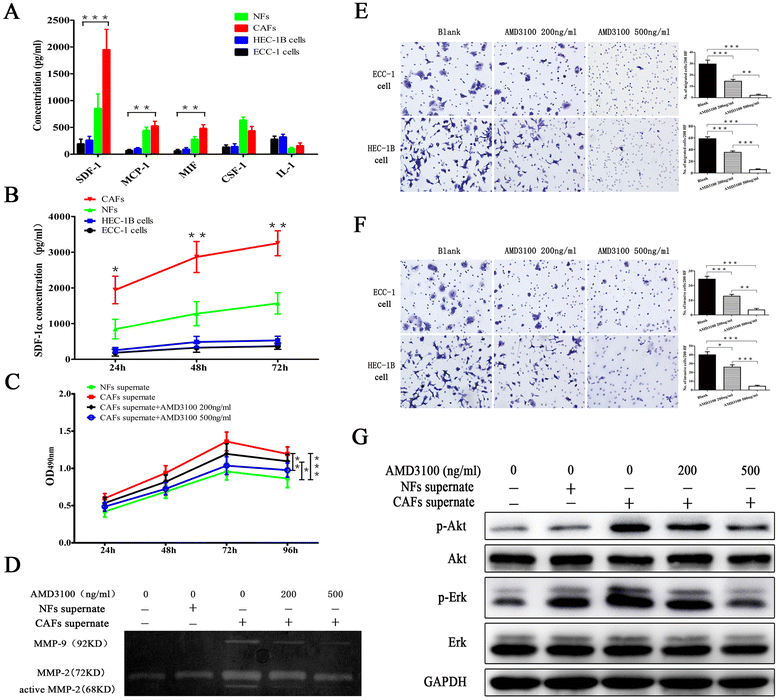
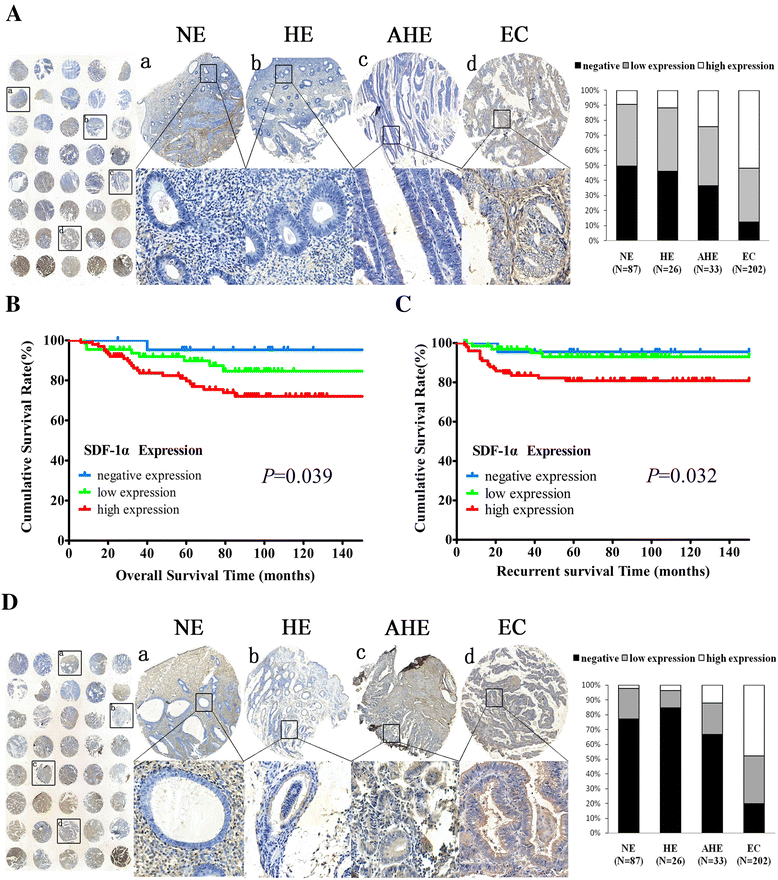
Methods: Stromal fibroblasts were isolated from endometrioid adenocarcinomas and normal endometrial tissues. The conditioned media of cultured CAFs and normal fibroblasts (NFs) were collected to detect the level of stromal cell-derived factor-1alpha (SDF-1α), macrophage chemoattractant protein-1 (MCP-1), migration inhibitory factor (MIF), colony stimulating factor-1 (CSF-1), and interleukin-1 (IL-1) by ELISA. The CAFs or NFs were cocultured with EC cell lines to determine the proliferation, migration, and invasion by MTT assays and transwell chambers. Xenograft models were used to observe tumor growth. Matrix metalloproteinases (MMP)-2 and MMP-9 activity was evaluated by zymography. AMD3100 (a chemokine receptor 4 (CXCR4) antagonist) was used to block the SDF-1/CXCR4 axis. Neutralizing antibodies were used to detect PI3K/Akt and MAPK/Erk pathways by western blotting. SDF-1α and CXCR4 expressions were analyzed in xenotransplanted tumors and 348 cases by immunohistochemistry.
Results: CAFs promoted proliferation, migration, and invasion as well as in vivo tumorigenesis of admixed EC cells significantly more than NFs by secreting SDF-1α. These effects were significantly inhibited by AMD3100. CAFs promoted EC progression via the SDF-1α/CXCR4 axis to activate the PI3K/Akt and MAPK/Erk signalings in a paracrine-dependent manner or increase MMP-2 and MMP-9 secretion in an autocrine-dependent manner. SDF-1α and CXCR4 expression upregulation accompanied clinical EC development and progression. High SDF-1α expression levels were associated with deep myometrial invasion, lymph node metastasis, and poor prognosis in EC.
Conclusions: Our data indicated that CAFs derived from EC tissues promoted EC progression via the SDF-1/CXCR4 axis in a paracrine- or autocrine-dependent manner. SDF-1α is a novel independent poor prognostic factor for EC patients' survival. Targeting the SDF-1/CXCR4 axis might provide a novel therapeutic strategy for EC treatment.
Figures
References
-
- Orimo A, Tomioka Y, Shimizu Y, Sato M, Oigawa S, Kamata K, et al. Cancer-associated myofibroblasts possess various factors to promote endometrial tumor progression. Clin Cancer Res. 2001;7(10):3097–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

