Evaluation of short message service and peer navigation to improve engagement in HIV care in South Africa: study protocol for a three-arm cluster randomized controlled trial
- PMID: 26852237
- PMCID: PMC4744624
- DOI: 10.1186/s13063-016-1190-y
Evaluation of short message service and peer navigation to improve engagement in HIV care in South Africa: study protocol for a three-arm cluster randomized controlled trial
Abstract
Background: In countries with a high burden of HIV, such as South Africa, where the epidemic remains the world's largest, improving early uptake of and consistent adherence to antiretroviral therapy could bring substantial HIV prevention gains. However, patients are not linked to or retained in care at rates needed to curtail the epidemic. Two strategies that have demonstrated a potential to stem losses along the HIV care cascade in the sub-Saharan African context are use of text messaging or short message service (SMS) and peer-navigation services.
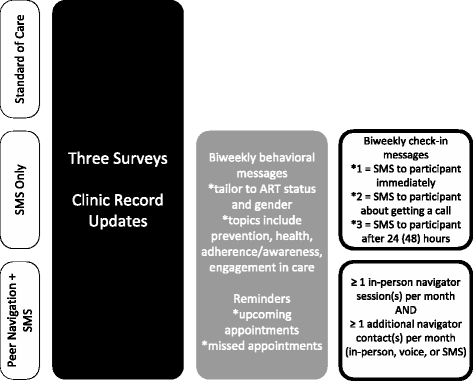
Methods/design: We designed a cluster randomized trial to assess the efficacy of an SMS intervention and a peer-navigation intervention to improve retention in care and treatment, timely linkage to care and treatment, medication adherence, and prevention behaviors in South Africa. Eighteen primary and community healthcare clinics in Rustenburg and Moses Kotane Sub-districts in the North West Province were randomized to one of three conditions: SMS intervention (n = 7), peer navigation intervention (n = 7), or standard of care (n = 4). Approximately 42 participants are being recruited at each clinic, which will result in a target of 750 participants. Eligible participants include patients accessing HIV testing or care in a study clinic, recently diagnosed with HIV, aged 18 years or older, and with access to a cellular telephone where they are willing to receive automated SMS with HIV-related messaging. Data collection includes extraction of visit information from clinical files and participant surveys at baseline, 6 months, and 12 months. Intent-to-treat (ITT) analysis will explore differences between randomization arms and the primary outcome of patient retention in care at 12 months following enrollment. We will also explore secondary outcomes including participants' a) timely linkage to care (within 3 months of HIV diagnosis), b) adherence to treatment based on self-report and clinic's medication dispensation dates, and c) condom-use behaviors.
Discussion: The findings will allow us to compare the efficacy of two complementary interventions, one that requires fewer resources to implement (SMS) and one (peer navigation) that offers more flexibility in terms of the patient barriers to care that it can address.
Trial registration: NCT02417233, registered 12 December 2014.
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

