Osteocyte Apoptosis Caused by Hindlimb Unloading is Required to Trigger Osteocyte RANKL Production and Subsequent Resorption of Cortical and Trabecular Bone in Mice Femurs
- PMID: 26852281
- PMCID: PMC5488280
- DOI: 10.1002/jbmr.2807
Osteocyte Apoptosis Caused by Hindlimb Unloading is Required to Trigger Osteocyte RANKL Production and Subsequent Resorption of Cortical and Trabecular Bone in Mice Femurs
Abstract
Osteocyte apoptosis is essential to activate bone remodeling in response to fatigue microdamage and estrogen withdrawal, such that apoptosis inhibition in vivo prevents the onset of osteoclastic resorption. Osteocyte apoptosis has also been spatially linked to bone resorption owing to disuse, but whether apoptosis plays a similar controlling role is unclear. We, therefore, 1) evaluated the spatial and temporal effects of disuse from hindlimb unloading (HLU) on osteocyte apoptosis, receptor activator of NF-κB ligand (RANKL) expression, bone resorption, and loss in mouse femora, and 2) tested whether osteocyte apoptosis was required to activate osteoclastic activity in cortical and trabecular bone by treating animals subjected to HLU with the pan-caspase apoptosis inhibitor, QVD (quinolyl-valyl-O-methylaspartyl-[-2,6-difluorophenoxy]-methylketone). Immunohistochemistry was used to identify apoptotic and RANKL-producing osteocytes in femoral diaphysis and distal trabecular bone, and µCT was used to determine the extent of trabecular bone loss owing to HLU. In both cortical and trabecular bone, 5 days of HLU increased osteocyte apoptosis significantly (3- and 4-fold, respectively, p < 0.05 versus Ctrl). At day 14, the apoptotic osteocyte number in femoral cortices declined to near control levels but remained elevated in trabeculae (3-fold versus Ctrl, p < 0.05). The number of osteocytes producing RANKL in both bone compartments was also significantly increased at day 5 of HLU (>1.5-fold versus Ctrl, p < 0.05) and further increased by day 14. Increases in osteocyte apoptosis and RANKL production preceded increases in bone resorption at both endocortical and trabecular surfaces. QVD completely inhibited not only the HLU-triggered increases in osteocyte apoptosis but also RANKL production and activation of bone resorption at both sites. Finally, µCT studies revealed that apoptosis inhibition completely prevented the trabecular bone loss caused by HLU. Together these data indicate that osteocyte apoptosis plays a central and controlling role in triggering osteocyte RANKL production and the activation of new resorption leading to bone loss in disuse. © 2016 American Society for Bone and Mineral Research.
© 2016 American Society for Bone and Mineral Research.
Conflict of interest statement
All authors state that they have no conflicts of interest.
Figures

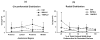
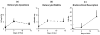

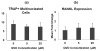
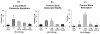

Similar articles
-
Osteocyte apoptosis and control of bone resorption following ovariectomy in mice.Bone. 2010 Mar;46(3):577-83. doi: 10.1016/j.bone.2009.11.006. Epub 2009 Nov 17. Bone. 2010. PMID: 19925896 Free PMC article.
-
Pannexin-1 and P2X7-Receptor Are Required for Apoptotic Osteocytes in Fatigued Bone to Trigger RANKL Production in Neighboring Bystander Osteocytes.J Bone Miner Res. 2016 Apr;31(4):890-9. doi: 10.1002/jbmr.2740. Epub 2016 Jan 20. J Bone Miner Res. 2016. PMID: 26553756 Free PMC article.
-
Inhibition of osteocyte apoptosis prevents the increase in osteocytic receptor activator of nuclear factor κB ligand (RANKL) but does not stop bone resorption or the loss of bone induced by unloading.J Biol Chem. 2015 Jul 31;290(31):18934-42. doi: 10.1074/jbc.M115.642090. Epub 2015 Jun 17. J Biol Chem. 2015. PMID: 26085098 Free PMC article.
-
Osteocyte control of bone remodeling: is sclerostin a key molecular coordinator of the balanced bone resorption-formation cycles?Osteoporos Int. 2014 Dec;25(12):2685-700. doi: 10.1007/s00198-014-2808-0. Epub 2014 Jul 17. Osteoporos Int. 2014. PMID: 25030653 Review.
-
Osteocytes and Paget's Disease of Bone.Curr Osteoporos Rep. 2024 Apr;22(2):266-272. doi: 10.1007/s11914-024-00863-5. Epub 2024 Mar 8. Curr Osteoporos Rep. 2024. PMID: 38457001 Free PMC article. Review.
Cited by
-
Regional differences in oxidative metabolism and mitochondrial activity among cortical bone osteocytes.Bone. 2016 Sep;90:15-22. doi: 10.1016/j.bone.2016.05.011. Epub 2016 May 31. Bone. 2016. PMID: 27260646 Free PMC article.
-
Acupotomy ameliorates subchondral bone absorption and mechanical properties in rabbits with knee osteoarthritis by regulating bone morphogenetic protein 2-Smad1 pathway.J Tradit Chin Med. 2023 Aug;43(4):734-743. doi: 10.19852/j.cnki.jtcm.20230404.001. J Tradit Chin Med. 2023. PMID: 37454258 Free PMC article.
-
The osteocyte as a signaling cell.Physiol Rev. 2022 Jan 1;102(1):379-410. doi: 10.1152/physrev.00043.2020. Epub 2021 Aug 2. Physiol Rev. 2022. PMID: 34337974 Free PMC article. Review.
-
Bone remodeling and cortical thinning distal to the femoral stem: a retrospective review.Arch Orthop Trauma Surg. 2023 Oct;143(10):6461-6467. doi: 10.1007/s00402-023-04860-8. Epub 2023 Apr 13. Arch Orthop Trauma Surg. 2023. PMID: 37055631
-
New Advances in Osteocyte Mechanotransduction.Curr Osteoporos Rep. 2021 Feb;19(1):101-106. doi: 10.1007/s11914-020-00650-y. Epub 2021 Jan 9. Curr Osteoporos Rep. 2021. PMID: 33420631 Free PMC article. Review.
References
-
- Gu G, Mulari M, Peng Z, Hentunen TA, Väänänen HK. Death of osteocytes turns off the inhibition of osteoclasts and triggers local bone resorption. Biochem Biophys Res Commun. 2005;335(4):1095–101. - PubMed
-
- Kogianni G, Mann V, Noble BS. Apoptotic bodies convey activity capable of initiating osteoclastogenesis and localized bone destruction. J Bone Miner Res. 2008;23(6):915–27. - PubMed
-
- Burr DB, Martin RB, Schaffler MB, Radin EL. Bone remodeling in response to in vivo fatigue microdamage. J Biomech. 1985;18(3):189–200. - PubMed
-
- Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB. Intracortical remodeling in adult rat long bones after fatigue loading. Bone. 1998;23(3):275–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

