Amomum tsao-ko fruit extract suppresses lipopolysaccharide-induced inducible nitric oxide synthase by inducing heme oxygenase-1 in macrophages and in septic mice
- PMID: 26852687
- PMCID: PMC4744820
- DOI: 10.1111/iep.12159
Amomum tsao-ko fruit extract suppresses lipopolysaccharide-induced inducible nitric oxide synthase by inducing heme oxygenase-1 in macrophages and in septic mice
Abstract
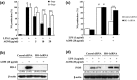
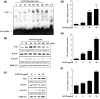
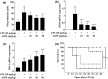
Amomum tsao-ko Crevost et Lemarié (Zingiberaceae) has traditionally been used to treat inflammatory and infectious diseases, such as throat infections, malaria, abdominal pain and diarrhoea. This study was designed to assess the anti-inflammatory effects and the molecular mechanisms of the methanol extract of A. tsao-ko (AOM) in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages and in a murine model of sepsis. In LPS-induced RAW 264.7 macrophages, AOM reduced the production of nitric oxide (NO) by inhibiting inducible nitric oxide synthase (iNOS) expression, and increased heme oxygenase-1 (HO-1) expression at the protein and mRNA levels. Pretreatment with SnPP (a selective inhibitor of HO-1) and silencing HO-1 using siRNA prevented the AOM-mediated inhibition of NO production and iNOS expression. Furthermore, AOM increased the expression and nuclear accumulation of NF-E2-related factor 2 (Nrf2), which enhanced Nrf2 binding to antioxidant response element (ARE). In addition, AOM induced the phosphorylation of extracellular regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) and generated reactive oxygen species (ROS). Furthermore, pretreatment with N-acetyl-l-cysteine (NAC; a ROS scavenger) diminished the AOM-induced phosphorylation of ERK and JNK and AOM-induced HO-1 expression, suggesting that ERK and JNK are downstream mediators of ROS during the AOM-induced signalling of HO-1 expression. In LPS-induced endotoxaemic mice, pretreatment with AOM reduced NO serum levels and liver iNOS expression and increased HO-1 expression and survival rates. These results indicate that AOM strongly inhibits LPS-induced NO production by activating the ROS/MAPKs/Nrf2-mediated HO-1 signalling pathway, and supports its pharmacological effects on inflammatory diseases.
Keywords: Amomum tsao-ko; HO-1; NO; Nrf2; ROS; sepsis.
© 2016 The Authors. International Journal of Experimental Pathology © 2016 International Journal of Experimental Pathology.
Figures



Similar articles
-
Amomum tsao-ko suppresses lipopolysaccharide-induced inflammatory responses in RAW264.7 macrophages via Nrf2-dependent heme oxygenase-1 expression.Am J Chin Med. 2014;42(5):1229-44. doi: 10.1142/S0192415X14500773. Epub 2014 Sep 1. Am J Chin Med. 2014. PMID: 25178279
-
7-Methoxy-(9H-β-Carbolin-1-il)-(E)-1-Propenoic Acid, a β-Carboline Alkaloid From Eurycoma longifolia, Exhibits Anti-Inflammatory Effects by Activating the Nrf2/Heme Oxygenase-1 Pathway.J Cell Biochem. 2016 Mar;117(3):659-70. doi: 10.1002/jcb.25315. Epub 2015 Sep 3. J Cell Biochem. 2016. PMID: 26291957
-
Anti-inflammatory action of methanol extract of Carthamus tinctorius involves in heme oxygenase-1 induction.J Ethnopharmacol. 2011 Jan 27;133(2):524-30. doi: 10.1016/j.jep.2010.10.029. Epub 2010 Oct 20. J Ethnopharmacol. 2011. PMID: 20969944
-
Induction of inducible nitric oxide synthase and heme oxygenase-1 in rat glial cells.Life Sci. 1998;62(17-18):1717-21. doi: 10.1016/s0024-3205(98)00134-9. Life Sci. 1998. PMID: 9585163 Review.
-
[New paradigm of host defense against intracellular pathogens by nitric oxide].Nihon Hansenbyo Gakkai Zasshi. 2009 Feb;78(1):41-7. doi: 10.5025/hansen.78.41. Nihon Hansenbyo Gakkai Zasshi. 2009. PMID: 19227148 Review. Japanese.
Cited by
-
An ethnopharmacological study of aromatic Uyghur medicinal plants in Xinjiang, China.Pharm Biol. 2017 Dec;55(1):1114-1130. doi: 10.1080/13880209.2016.1270971. Pharm Biol. 2017. PMID: 28209076 Free PMC article.
-
Monoterpenoids from the Fruits of Amomum tsao-ko Have Inhibitory Effects on Nitric Oxide Production.Plants (Basel). 2021 Jan 28;10(2):257. doi: 10.3390/plants10020257. Plants (Basel). 2021. PMID: 33525660 Free PMC article.
-
Identification of in vitro-in vivo components of Caoguo using accelerated solvent extraction combined with gas chromatography-mass spectrometry integrated with network pharmacology on indigestion.Ann Transl Med. 2021 Aug;9(15):1247. doi: 10.21037/atm-21-3245. Ann Transl Med. 2021. PMID: 34532384 Free PMC article.
-
Effect of Yikou-Sizi powder hot compress on gastrointestinal functional recovery in patients after abdominal surgery: Study protocol for a randomized controlled trial.Medicine (Baltimore). 2018 Sep;97(38):e12438. doi: 10.1097/MD.0000000000012438. Medicine (Baltimore). 2018. PMID: 30235726 Free PMC article.
-
Health-promoting compounds in Amomum villosum Lour and Amomum tsao-ko: Fruit essential oil exhibiting great potential for human health.Heliyon. 2024 Mar 3;10(5):e27492. doi: 10.1016/j.heliyon.2024.e27492. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38463888 Free PMC article. Review.
References
-
- Chen T.H., Hsu Y.T., Chen C.H., Kao S.H. & Lee H.M. (2007) Tanshinone IIA from Salvia miltiorrhiza induces heme oxygenase‐1 expression and inhibits lipopolysaccharide‐induced nitric oxide expression in RAW 264.7 cells. Mitochondrion 7, 101–105. - PubMed
-
- Cheung K.L., Lee J.H., Shu L., Kim J.H., Sacks D.B. & Kong A.N. (2013) The Ras GTPase‐activating‐like protein IQGAP1 mediates Nrf2 protein activation via the mitogen‐activated protein kinase/extracellular signal‐regulated kinase (ERK) kinase (MEK)‐ERK pathway. J. Biol. Chem. 288, 22378–22386. - PMC - PubMed
-
- Dinarello C.A. (1997) Proinflammatory and anti‐inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest 112, 321S–329S. - PubMed
-
- Fubini B. & Hubbard A. (2003) Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic. Biol. Med. 34, 1507–1516. - PubMed
-
- Gozzelino R., Jeney V. & Soares M.P. (2010) Mechanisms of cell protection by heme oxygenase‐1. Annu. Rev. Pharmacol. Toxicol. 50, 323–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

