Mutant bacterial sodium channels as models for local anesthetic block of eukaryotic proteins
- PMID: 26852716
- PMCID: PMC4954582
- DOI: 10.1080/19336950.2016.1148224
Mutant bacterial sodium channels as models for local anesthetic block of eukaryotic proteins
Abstract
Voltage gated sodium channels are the target of a range of local anesthetic, anti-epileptic and anti-arrhythmic compounds. But, gaining a molecular level understanding of their mode of action is difficult as we only have atomic resolution structures of bacterial sodium channels not their eukaryotic counterparts. In this study we used molecular dynamics simulations to demonstrate that the binding sites of both the local anesthetic benzocaine and the anti-epileptic phenytoin to the bacterial sodium channel NavAb can be altered significantly by the introduction of point mutations. Free energy techniques were applied to show that increased aromaticity in the pore of the channel, used to emulate the aromatic residues observed in eukaryotic Nav1.2, led to changes in the location of binding and dissociation constants of each drug relative to wild type NavAb. Further, binding locations and dissociation constants obtained for both benzocaine (660 μM) and phenytoin (1 μM) in the mutant channels were within the range expected from experimental values obtained from drug binding to eukaryotic sodium channels, indicating that these mutant NavAb may be a better model for drug binding to eukaryotic channels than the wild type.
Keywords: NavAb; benzocaine; drug binding; local anesthetic; phenytoin; sodium channel.
Figures


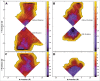
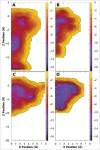


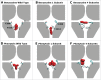
Comment in
-
Germs against pain: Humanized bacterial sodium channels.Channels (Austin). 2016 Nov;10(6):1. doi: 10.1080/19336950.2016.1205435. Epub 2016 Jun 24. Channels (Austin). 2016. PMID: 27341662 Free PMC article. No abstract available.
Similar articles
-
Local anesthetic and antiepileptic drug access and binding to a bacterial voltage-gated sodium channel.Proc Natl Acad Sci U S A. 2014 Sep 9;111(36):13057-62. doi: 10.1073/pnas.1408710111. Epub 2014 Aug 18. Proc Natl Acad Sci U S A. 2014. PMID: 25136136 Free PMC article.
-
Fenestrations control resting-state block of a voltage-gated sodium channel.Proc Natl Acad Sci U S A. 2018 Dec 18;115(51):13111-13116. doi: 10.1073/pnas.1814928115. Epub 2018 Dec 5. Proc Natl Acad Sci U S A. 2018. PMID: 30518562 Free PMC article.
-
Architecture and pore block of eukaryotic voltage-gated sodium channels in view of NavAb bacterial sodium channel structure.Mol Pharmacol. 2012 Jul;82(1):97-104. doi: 10.1124/mol.112.078212. Epub 2012 Apr 13. Mol Pharmacol. 2012. PMID: 22505150
-
Pharmacological insights and quirks of bacterial sodium channels.Handb Exp Pharmacol. 2014;221:251-67. doi: 10.1007/978-3-642-41588-3_12. Handb Exp Pharmacol. 2014. PMID: 24737240 Review.
-
Voltage-gated Sodium Channels and Blockers: An Overview and Where Will They Go?Curr Med Sci. 2019 Dec;39(6):863-873. doi: 10.1007/s11596-019-2117-0. Epub 2019 Dec 16. Curr Med Sci. 2019. PMID: 31845216 Review.
Cited by
-
Noncanonical Ion Channel Behaviour in Pain.Int J Mol Sci. 2019 Sep 15;20(18):4572. doi: 10.3390/ijms20184572. Int J Mol Sci. 2019. PMID: 31540178 Free PMC article. Review.
-
Germs against pain: Humanized bacterial sodium channels.Channels (Austin). 2016 Nov;10(6):1. doi: 10.1080/19336950.2016.1205435. Epub 2016 Jun 24. Channels (Austin). 2016. PMID: 27341662 Free PMC article. No abstract available.
-
Physical basis of specificity and delayed binding of a subtype selective sodium channel inhibitor.Sci Rep. 2018 Jan 22;8(1):1356. doi: 10.1038/s41598-018-19850-9. Sci Rep. 2018. PMID: 29358762 Free PMC article.
-
Phenytoin: a step by step insight into its multiple mechanisms of action-80 years of mechanistic studies in neuropharmacology.J Neurol. 2017 Sep;264(9):2043-2047. doi: 10.1007/s00415-017-8465-4. Epub 2017 Mar 27. J Neurol. 2017. PMID: 28349209 No abstract available.
-
Drugs exhibit diverse binding modes and access routes in the Nav1.5 cardiac sodium channel pore.J Gen Physiol. 2025 Mar 3;157(2):e202413658. doi: 10.1085/jgp.202413658. Epub 2025 Jan 7. J Gen Physiol. 2025. PMID: 39774837
References
-
- Catterall WA, Goldin AL, Waxman SG. International union of pharmacology. XLVII. nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev 2005; 57:397-409; PMID:16382098; http://dx.doi.org/10.1124/pr.57.4.4 - DOI - PubMed
-
- Goldin AL, Barchi RL, Caldwell JH, Hofmann JH, Howe JR, Hunter JC, Kallen RG, Mandel G., Meisler MH, Netter YB, et al.. Nomenclature of voltage-gated sodium channels. Neuron 2000; 28:365-368; PMID:11144347; http://dx.doi.org/10.1016/S0896-6273(00)00116-1 - DOI - PubMed
-
- Amd N, Liu YR, Priori SG. Sodium channel mutations and arrhythmias. Nat Rev Cardiol 6:337-348; PMID:19377496 - PubMed
-
- Roden DM, George AL. The cardiac ion channels: relevance to management of arrhythmias. Annu Rev Med 1996; 47:135-148; PMID:8712768; http://dx.doi.org/10.1146/annurev.med.47.1.135 - DOI - PubMed
-
- Waxman SG, Hains BC. Fire and phantoms after spinal cord injury: Na+ channels and central pain. Trends Neurosci 2006; 29:207-215; PMID:16494954; http://dx.doi.org/10.1016/j.tins.2006.02.003 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
