Thioetherification via Photoredox/Nickel Dual Catalysis
- PMID: 26852821
- PMCID: PMC4854196
- DOI: 10.1021/acs.orglett.6b00208
Thioetherification via Photoredox/Nickel Dual Catalysis
Abstract
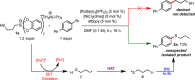
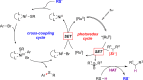
Hypervalent alkylsilicates represent new and readily accessible precursors for the generation of alkyl radicals under photoredox conditions. Alkyl radicals generated from such silicates serve as effective hydrogen atom abstractors from thiols, furnishing thiyl radicals. The reactive sulfur species generated in this manner can be funneled into a nickel-mediated cross-coupling cycle employing aromatic bromides to furnish thioethers. The serendipitous discovery of this reaction and its utilization for the thioetherification of various aryl and heteroaryl bromides with a diverse array of thiols is described. The S-H selective H atom abstraction event enables a wide range of functional groups, including those bearing protic moieties, to be tolerated.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Base-Free Photoredox/Nickel Dual-Catalytic Cross-Coupling of Ammonium Alkylsilicates.J Am Chem Soc. 2016 Jan 20;138(2):475-8. doi: 10.1021/jacs.5b10963. Epub 2015 Dec 24. J Am Chem Soc. 2016. PMID: 26704168 Free PMC article.
-
Engaging Alkenyl Halides with Alkylsilicates via Photoredox Dual Catalysis.Org Lett. 2016 Feb 19;18(4):764-7. doi: 10.1021/acs.orglett.6b00024. Epub 2016 Feb 1. Org Lett. 2016. PMID: 26828317 Free PMC article.
-
Single-Electron Transmetalation via Photoredox/Nickel Dual Catalysis: Unlocking a New Paradigm for sp(3)-sp(2) Cross-Coupling.Acc Chem Res. 2016 Jul 19;49(7):1429-39. doi: 10.1021/acs.accounts.6b00214. Epub 2016 Jul 5. Acc Chem Res. 2016. PMID: 27379472 Free PMC article.
-
Photoredox-Mediated Routes to Radicals: The Value of Catalytic Radical Generation in Synthetic Methods Development.ACS Catal. 2017 Apr 7;7(4):2563-2575. doi: 10.1021/acscatal.7b00094. Epub 2017 Mar 14. ACS Catal. 2017. PMID: 28413692 Free PMC article. Review.
-
Ni-catalyzed C-S bond construction and cleavage.Chem Soc Rev. 2022 Oct 3;51(19):8351-8377. doi: 10.1039/d2cs00553k. Chem Soc Rev. 2022. PMID: 36107012 Review.
Cited by
-
Selective formation of heteroaryl thioethers via a phosphonium ion coupling reaction.Tetrahedron. 2018 Jun 21;74(25):3129-3136. doi: 10.1016/j.tet.2017.12.040. Epub 2017 Dec 21. Tetrahedron. 2018. PMID: 30479455 Free PMC article.
-
1,4-Dihydropyridines as Alkyl Radical Precursors: Introducing the Aldehyde Feedstock to Nickel/Photoredox Dual Catalysis.ACS Catal. 2016 Dec 2;6(12):8004-8008. doi: 10.1021/acscatal.6b02786. Epub 2016 Oct 27. ACS Catal. 2016. PMID: 27990318 Free PMC article.
-
Visible-Light-Promoted C-S Cross-Coupling Reaction for Synthesis of Aromatic Thioethers.Organic Synth. 2023;100:234-247. doi: 10.15227/orgsyn.100.0234. Epub 2023 Jun 15. Organic Synth. 2023. PMID: 38152293 Free PMC article.
-
Photoredox Catalysis in Organic Chemistry.J Org Chem. 2016 Aug 19;81(16):6898-926. doi: 10.1021/acs.joc.6b01449. Epub 2016 Aug 1. J Org Chem. 2016. PMID: 27477076 Free PMC article.
-
Dual Catalysis Strategies in Photochemical Synthesis.Chem Rev. 2016 Sep 14;116(17):10035-74. doi: 10.1021/acs.chemrev.6b00018. Epub 2016 Apr 25. Chem Rev. 2016. PMID: 27109441 Free PMC article. Review.
References
-
- Douglas J. J.; Nguyen J. D.; Cole K. P.; Stephenson C. R. J. Aldrichimica Acta 2014, 47, 15.
- Tucker J. W.; Stephenson C. R. J. J. Org. Chem. 2012, 77, 1617.10.1021/jo202538x. - DOI - PubMed
- Prier C. K.; Rankic D. A.; MacMillan D. W. C. Chem. Rev. 2013, 113, 5322.10.1021/cr300503r. - DOI - PMC - PubMed
- Hopkinson M. N.; Sahoo B.; Li J.-L.; Glorius F. Chem. - Eur. J. 2014, 20, 3874.10.1002/chem.201304823. - DOI - PubMed
-
-
Seminal reports:
- Tellis J. C.; Primer D. N.; Molander G. A. Science 2014, 345, 433.10.1126/science.1253647. - DOI - PMC - PubMed
- Zuo Z. W.; Ahneman D. T.; Chu L. L.; Terrett J. A.; Doyle A. G.; MacMillan D. W. C. Science 2014, 345, 437.10.1126/science.1255525. - DOI - PMC - PubMed
- Kalyani D.; McMurtrey K. B.; Neufeldt S. R.; Sanford M. S. J. Am. Chem. Soc. 2011, 133, 18566.10.1021/ja208068w. - DOI - PMC - PubMed
- Ye Y.; Sanford M. S. J. Am. Chem. Soc. 2012, 134, 9034.10.1021/ja301553c. - DOI - PMC - PubMed
- Sahoo B.; Hopkinson M. N.; Glorius F. J. Am. Chem. Soc. 2013, 135, 5505.10.1021/ja400311h. - DOI - PubMed
-
-
-
Recent work in photoredox dual catalysis:
- Primer D. N.; Karakaya I.; Tellis J. C.; Molander G. A. J. Am. Chem. Soc. 2015, 137, 2195.10.1021/ja512946e. - DOI - PMC - PubMed
- Karakaya I.; Primer D. N.; Molander G. A. Org. Lett. 2015, 17, 3294.10.1021/acs.orglett.5b01463. - DOI - PMC - PubMed
- El Khatib M.; Serafim R. A. M.; Molander G. A. Angew. Chem., Int. Ed. 2016, 55, 254.10.1002/anie.201506147. - DOI - PMC - PubMed
- Rueping M.; Koenigs R. M.; Poscharny K.; Fabry D. C.; Leonori D.; Vila C. Chem. - Eur. J. 2012, 18, 5170.10.1002/chem.201200050. - DOI - PubMed
- Shu X. Z.; Zhang M.; He Y.; Frei H.; Toste F. D. J. Am. Chem. Soc. 2014, 136, 5844.10.1021/ja500716j. - DOI - PMC - PubMed
- Noble A.; McCarver S. J.; MacMillan D. W. C. J. Am. Chem. Soc. 2015, 137, 624.10.1021/ja511913h. - DOI - PMC - PubMed
- Chu L.; Lipshultz J. M.; MacMillan D. W. C. Angew. Chem., Int. Ed. 2015, 54, 7929.10.1002/anie.201501908. - DOI - PMC - PubMed
- Le C. C.; MacMillan D. W. C. J. Am. Chem. Soc. 2015, 137, 11938.10.1021/jacs.5b08304. - DOI - PMC - PubMed
-
-
- Jouffroy M.; Primer D. N.; Molander G. A. J. Am. Chem. Soc. 2016, 138, 475.10.1021/jacs.5b10963. - DOI - PMC - PubMed
- Corce V.; Chamoreau L.-M.; Derat E.; Goddard J.-P.; Ollivier C.; Fensterbank L. Angew. Chem., Int. Ed. 2015, 54, 11414.10.1002/anie.201504963. - DOI - PubMed
- Matsuoka D.; Nishigaichi Y. Chem. Lett. 2014, 43, 559.10.1246/cl.131132. - DOI
- Matsuoka D.; Nishigaichi Y. Chem. Lett. 2015, 44, 163.10.1246/cl.140940. - DOI
-
- Tian Z.; Fattahi A.; Lis L.; Kass S. R. J. Am. Chem. Soc. 2006, 128, 17087.10.1021/ja065348u. - DOI - PubMed
- Blanksby S. J.; Ellison G. B. Acc. Chem. Res. 2003, 36, 255.10.1021/ar020230d. - DOI - PubMed
- Huston P.; Espenson J. H.; Bakac A. J. Am. Chem. Soc. 1992, 114, 9510.10.1021/ja00050a033. - DOI
- Review on thiyl radicals:Denes F.; Pichowicz M.; Povie G.; Renaud P. Chem. Rev. 2014, 114, 2587.10.1021/cr400441m. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

