The novel adipokine progranulin counteracts IL-1 and TLR4-driven inflammatory response in human and murine chondrocytes via TNFR1
- PMID: 26853108
- PMCID: PMC4745010
- DOI: 10.1038/srep20356
The novel adipokine progranulin counteracts IL-1 and TLR4-driven inflammatory response in human and murine chondrocytes via TNFR1
Abstract
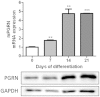
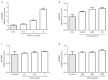
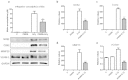
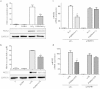
Progranulin (PGRN) is a recently identified adipokine that is supposed to have anti-inflammatory actions. The proinflammatory cytokine interleukin-1β (IL1β) stimulates several mediators of cartilage degradation. Toll like receptor-4 (TLR4) can bind to various damage-associated molecular patterns, leading to inflammatory condition. So far, no data exist of PGRN effects in inflammatory conditions induced by IL1β or lipopolysaccharide (LPS). Here, we investigated the anti-inflammatory potential of PGRN in IL1β- or LPS-induced inflammatory responses of chondrocytes. Human osteoarthritic chondrocytes and ATDC-5 cells were treated with PGRN in presence or not of IL1β or LPS. First, we showed that recombinant PGRN had no effects on cell viability. We present evidence that PGRN expression was increased during the differentiation of ATDC-5 cell line. Moreover, PGRN mRNA and protein expression is increased in cartilage, synovial and infrapatellar fat pad tissue samples from OA patients. PGRN mRNA levels are upregulated under TNFα and IL1β stimulation. Our data showed that PGRN is able to significantly counteract the IL1β-induced expression of NOS2, COX2, MMP13 and VCAM-1. LPS-induced expression of NOS2 is also decreased by PGRN. These effects are mediated, at least in part, through TNFR1. Taken together, our results suggest that PGRN has a clear anti-inflammatory function.
Figures

References
-
- Goldring M. B. & Goldring S. R. Osteoarthritis. J Cell Physiol 213, 626–634 (2007). - PubMed
-
- Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage 21, 16–21 (2013). - PubMed
-
- Gómez R. et al. What’s new in our understanding of the role of adipokines in rheumatic diseases? Nat Rev Rheumatol 7, 528–36 (2011). - PubMed
-
- Lago F., Dieguez C., Gómez-Reino J. & Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol 3, 716–24 (2007). - PubMed
-
- Conde J. et al. Expanding the adipokine network in cartilage: identification and regulation of novel factors in human and murine chondrocytes. Ann Rheum Dis 70, 551–559 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

