Mechanical evidence that Australopithecus sediba was limited in its ability to eat hard foods
- PMID: 26853550
- PMCID: PMC4748115
- DOI: 10.1038/ncomms10596
Mechanical evidence that Australopithecus sediba was limited in its ability to eat hard foods
Abstract
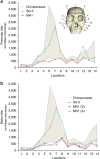
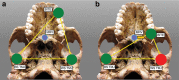
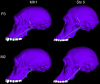
Australopithecus sediba has been hypothesized to be a close relative of the genus Homo. Here we show that MH1, the type specimen of A. sediba, was not optimized to produce high molar bite force and appears to have been limited in its ability to consume foods that were mechanically challenging to eat. Dental microwear data have previously been interpreted as indicating that A. sediba consumed hard foods, so our findings illustrate that mechanical data are essential if one aims to reconstruct a relatively complete picture of feeding adaptations in extinct hominins. An implication of our study is that the key to understanding the origin of Homo lies in understanding how environmental changes disrupted gracile australopith niches. Resulting selection pressures led to changes in diet and dietary adaption that set the stage for the emergence of our genus.
Figures


References
-
- Scott R. S., Ungar P. S., Bergstrom T. S. & Brown C. A. Dental microwear texture analysis shows within-species diet variability in fossil hominins. Nature 436, 693–695 (2005). - PubMed
-
- Sponheimer M. et al.. Isotopic evidence for dietary variability in the early hominin Paranthropus robustus. Science 314, 980–982 (2006). - PubMed
-
- van der Merwe N. J., Masao F. T. & Bamford M. K. Isotopic evidence for contrasting diets of early hominins Homo habilis and Australopithecus boisei of Tanzania. S. Afr. J. Sci. 104, 153–155 (2008).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

