Exercise, inflammation, and fatigue in cancer survivors
- PMID: 26853557
- PMCID: PMC4755327
Exercise, inflammation, and fatigue in cancer survivors
Abstract
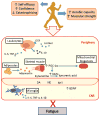
Cancer-related fatigue significantly disrupts normal functioning and quality of life for a substantial portion of cancer survivors, and may persist for years following cancer treatment. While the causes of persistent fatigue among cancer survivors are not yet fully understood, accumulating evidence suggests that several pathways, including chronic inflammation, autonomic imbalance, HPA-axis dysfunction, and/or mitochondrial damage, could contribute towards the disruption of normal neuronal function and result in the symptom of cancer-related fatigue. Exercise training interventions have been shown to be some of the more successful treatment options to address cancer-related fatigue. In this review, we discuss the literature regarding the causes of persistent fatigue in cancer survivors and the mechanisms by which exercise may relieve this symptom. There is still much work to be done until the prescription of exercise becomes standard practice for cancer survivors. With improvements in the quality of studies, evidenced-based exercise interventions will allow exercise scientists and oncologists to work together to treat cancer-related fatigue.
Keywords: Cancer; Exercise; Fatigue; Inflammation; Physical Activity.
Copyright © 2015 International Society of Exercise and Immunology. All rights reserved.
Figures

References
-
- Bennett B, et al. The experience of cancer-related fatigue and chronic fatigue syndrome: a qualitative and comparative study. J Pain Symptom Manage. 2007;34(2):126–35. - PubMed
-
- Cleeland CS, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97(11):2919–25. - PubMed
-
- Ganz PA, et al. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst. 2002;94(1):39–49. - PubMed
