Impact of high CO2 on the geochemistry of the coralline algae Lithothamnion glaciale
- PMID: 26853562
- PMCID: PMC4744931
- DOI: 10.1038/srep20572
Impact of high CO2 on the geochemistry of the coralline algae Lithothamnion glaciale
Abstract
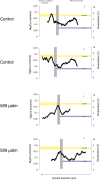
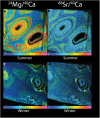
Coralline algae are a significant component of the benthic ecosystem. Their ability to withstand physical stresses in high energy environments relies on their skeletal structure which is composed of high Mg-calcite. High Mg-calcite is, however, the most soluble form of calcium carbonate and therefore potentially vulnerable to the change in carbonate chemistry resulting from the absorption of anthropogenic CO2 by the ocean. We examine the geochemistry of the cold water coralline alga Lithothamnion glaciale grown under predicted future (year 2050) high pCO2 (589 μatm) using Electron microprobe and NanoSIMS analysis. In the natural and control material, higher Mg calcite forms clear concentric bands around the algal cells. As expected, summer growth has a higher Mg content compared to the winter growth. In contrast, under elevated CO2 no banding of Mg is recognisable and overall Mg concentrations are lower. This reduction in Mg in the carbonate undermines the accuracy of the Mg/Ca ratio as proxy for past temperatures in time intervals with significantly different carbonate chemistry. Fundamentally, the loss of Mg in the calcite may reduce elasticity thereby changing the structural properties, which may affect the ability of L. glaciale to efficiently function as a habitat former in the future ocean.
Figures



References
-
- Foster M. S. Rhodoliths: between rocks and soft places Journal of Phycology 37, 659–667 (2001).
-
- Freiwald A. & Henrich R. Reefal coralline algal build-ups within the Arctic Circle: morphology and sedimentary dynamics under extreme environmental seasonality. Sedimentology 41, 963–984 (1994).
-
- Kamenos N. A., Moore P. G. & Hall-Spencer J. M. Nursey-area function of maerl groudns for juvenile queen scallops Aequipecten opercularis ad other invertebrates. Marine Ecology Progress Series 274, 183–189 (2004).
-
- Mackenzie F. T., Lerman A. & Andersson A. J. Past and present of sediment and carbon biogeochemical cycling models. Biogeosciences 1, 11–32 (2004).
-
- Martin S., Clavier, Chauvaud L. & Thouzeau G. Community metabolism in temperate maerl beds. I. Carbon and carbonate fluxes. Marine Ecology Progress Series 335, 19–29 (2007).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

