Extracellular matrix stiffness dictates Wnt expression through integrin pathway
- PMID: 26854061
- PMCID: PMC4745056
- DOI: 10.1038/srep20395
Extracellular matrix stiffness dictates Wnt expression through integrin pathway
Abstract
It is well established that extracellular matrix (ECM) stiffness plays a significant role in regulating the phenotypes and behaviors of many cell types. However, the mechanism underlying the sensing of mechanical cues and subsequent elasticity-triggered pathways remains largely unknown. We observed that stiff ECM significantly enhanced the expression level of several members of the Wnt/β-catenin pathway in both bone marrow mesenchymal stem cells and primary chondrocytes. The activation of β-catenin by stiff ECM is not dependent on Wnt signals but is elevated by the activation of integrin/ focal adhesion kinase (FAK) pathway. The accumulated β-catenin then bound to the wnt1 promoter region to up-regulate the gene transcription, thus constituting a positive feedback of the Wnt/β-catenin pathway. With the amplifying effect of positive feedback, this integrin-activated β-catenin/Wnt pathway plays significant roles in mediating the enhancement of Wnt signal on stiff ECM and contributes to the regulation of mesenchymal stem cell differentiation and primary chondrocyte phenotype maintenance. The present integrin-regulated Wnt1 expression and signaling contributes to the understanding of the molecular mechanisms underlying the regulation of cell behaviors by ECM elasticity.
Figures



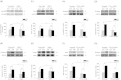

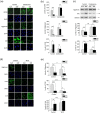

Similar articles
-
Apc bridges Wnt/β-catenin and BMP signaling during osteoblast differentiation of KS483 cells.Exp Cell Res. 2011 Jun 10;317(10):1411-21. doi: 10.1016/j.yexcr.2011.03.007. Epub 2011 Mar 22. Exp Cell Res. 2011. PMID: 21402068
-
Intermittent cyclic mechanical tension promotes endplate cartilage degeneration via canonical Wnt signaling pathway and E-cadherin/β-catenin complex cross-talk.Osteoarthritis Cartilage. 2016 Jan;24(1):158-68. doi: 10.1016/j.joca.2015.07.019. Epub 2015 Aug 3. Osteoarthritis Cartilage. 2016. PMID: 26247612
-
Fibroblast growth factor and canonical WNT/β-catenin signaling cooperate in suppression of chondrocyte differentiation in experimental models of FGFR signaling in cartilage.Biochim Biophys Acta. 2015 May;1852(5):839-50. doi: 10.1016/j.bbadis.2014.12.020. Epub 2015 Jan 2. Biochim Biophys Acta. 2015. PMID: 25558817
-
[Regulation of osteoblasts and chondrocytes by Wnt signaling.].Clin Calcium. 2019;29(3):299-307. doi: 10.20837/4201903299. Clin Calcium. 2019. PMID: 30814374 Review. Japanese.
-
Extracellular matrix stiffness and Wnt/β-catenin signaling in physiology and disease.Biochem Soc Trans. 2020 Jun 30;48(3):1187-1198. doi: 10.1042/BST20200026. Biochem Soc Trans. 2020. PMID: 32412078 Review.
Cited by
-
Articular Chondrocyte Phenotype Regulation through the Cytoskeleton and the Signaling Processes That Originate from or Converge on the Cytoskeleton: Towards a Novel Understanding of the Intersection between Actin Dynamics and Chondrogenic Function.Int J Mol Sci. 2021 Mar 23;22(6):3279. doi: 10.3390/ijms22063279. Int J Mol Sci. 2021. PMID: 33807043 Free PMC article. Review.
-
Control of innate immune response by biomaterial surface topography, energy, and stiffness.Acta Biomater. 2021 Oct 1;133:58-73. doi: 10.1016/j.actbio.2021.04.021. Epub 2021 Apr 18. Acta Biomater. 2021. PMID: 33882355 Free PMC article. Review.
-
Physical and mechanical cues affecting biomaterial-mediated plasmid DNA delivery: insights into non-viral delivery systems.J Genet Eng Biotechnol. 2021 Jun 17;19(1):90. doi: 10.1186/s43141-021-00194-3. J Genet Eng Biotechnol. 2021. PMID: 34142237 Free PMC article. Review.
-
Matrisome Transcriptome Dynamics during Tissue Aging.Life (Basel). 2024 May 7;14(5):593. doi: 10.3390/life14050593. Life (Basel). 2024. PMID: 38792614 Free PMC article.
-
Mechanically patterned neuromuscular junctions-in-a-dish have improved functional maturation.Mol Biol Cell. 2017 Jul 7;28(14):1950-1958. doi: 10.1091/mbc.E17-01-0046. Epub 2017 May 11. Mol Biol Cell. 2017. PMID: 28495800 Free PMC article.
References
-
- Discher D. E., Janmey P. & Wang Y. L. Tissue cells feel and respond to the stiffness of their substrate. Science. 310, 1139–1143 (2005). - PubMed
-
- Higuchi A., Ling Q.-D., Chang Y., Hsu S.-T. & Umezawa A. Physical Cues of Biomaterials Guide Stem Cell Differentiation Fate. Chem Rev. 113, 3297–328 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

