Circulating HMGB1 and RAGE as Clinical Biomarkers in Malignant and Autoimmune Diseases
- PMID: 26854151
- PMCID: PMC4665591
- DOI: 10.3390/diagnostics5020219
Circulating HMGB1 and RAGE as Clinical Biomarkers in Malignant and Autoimmune Diseases
Abstract
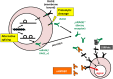
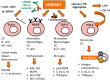
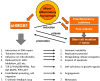
High molecular group box 1 (HMGB1) is a highly conserved member of the HMG-box-family; abundantly expressed in almost all human cells and released in apoptosis; necrosis or by activated immune cells. Once in the extracellular space, HMGB1 can act as a danger associated molecular pattern (DAMP), thus stimulating or inhibiting certain functions of the immune system; depending on the "combinatorial cocktail" of the surrounding milieu. HMGB1 exerts its various functions through binding to a multitude of membrane-bound receptors such as TLR-2; -4 and -9; IL-1 and RAGE (receptor for advanced glycation end products); partly complex-bound with intracellular fragments like nucleosomes. Soluble RAGE in the extracellular space, however, acts as a decoy receptor by binding to HMGB1 and inhibiting its effects. This review aims to outline today's knowledge of structure, intra- and extracellular functions including mechanisms of release and finally the clinical relevance of HMGB1 and RAGE as clinical biomarkers in therapy monitoring, prediction and prognosis of malignant and autoimmune disease.
Keywords: DAMP; HMBG1; RAGE; autoimmune disease; biomarkers; circulation; prognosis; therapy response; tumor.
Figures

Similar articles
-
An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies.Pharmacol Ther. 2014 Mar;141(3):347-57. doi: 10.1016/j.pharmthera.2013.11.001. Epub 2013 Nov 9. Pharmacol Ther. 2014. PMID: 24220159 Review.
-
RAGE and TLRs: relatives, friends or neighbours?Mol Immunol. 2013 Dec;56(4):739-44. doi: 10.1016/j.molimm.2013.07.008. Epub 2013 Aug 14. Mol Immunol. 2013. PMID: 23954397 Review.
-
Association of high mobility group BOX-1 and receptor for advanced glycation endproducts with clinicopathological features of haematological malignancies: a systematic review.Contemp Oncol (Pozn). 2016;20(6):425-429. doi: 10.5114/wo.2016.65600. Epub 2017 Jan 12. Contemp Oncol (Pozn). 2016. PMID: 28239277 Free PMC article. Review.
-
Targeting high mobility group box protein 1 ameliorates testicular inflammation in experimental autoimmune orchitis.Hum Reprod. 2015 Feb;30(2):417-31. doi: 10.1093/humrep/deu320. Epub 2014 Dec 1. Hum Reprod. 2015. PMID: 25452436
-
HMGB1: new biomarker and therapeutic target of autoimmune and autoinflammatory skin diseases.Front Immunol. 2025 Apr 16;16:1569632. doi: 10.3389/fimmu.2025.1569632. eCollection 2025. Front Immunol. 2025. PMID: 40308590 Free PMC article. Review.
Cited by
-
Plasma Concentrations of High Mobility Group Box 1 Proteins and Soluble Receptors for Advanced Glycation End-Products Are Relevant Biomarkers of Cognitive Impairment in Alcohol Use Disorder: A Pilot Study.Toxics. 2024 Feb 29;12(3):190. doi: 10.3390/toxics12030190. Toxics. 2024. PMID: 38535924 Free PMC article.
-
Increased GFAP concentrations in the cerebrospinal fluid of patients with unipolar depression.Transl Psychiatry. 2021 May 21;11(1):308. doi: 10.1038/s41398-021-01423-6. Transl Psychiatry. 2021. PMID: 34021122 Free PMC article.
-
Emerging role of HMGB1 in lung diseases: friend or foe.J Cell Mol Med. 2017 Jun;21(6):1046-1057. doi: 10.1111/jcmm.13048. Epub 2016 Dec 31. J Cell Mol Med. 2017. PMID: 28039939 Free PMC article. Review.
-
The Association of HMGB1 and RAGE Gene Polymorphisms with IgA Vasculitis.Biochem Genet. 2024 Jun;62(3):2268-2278. doi: 10.1007/s10528-023-10536-0. Epub 2023 Oct 30. Biochem Genet. 2024. PMID: 37902913
-
HMGB1 participates in LPS‑induced acute lung injury by activating the AIM2 inflammasome in macrophages and inducing polarization of M1 macrophages via TLR2, TLR4, and RAGE/NF‑κB signaling pathways.Int J Mol Med. 2020 Jan;45(1):61-80. doi: 10.3892/ijmm.2019.4402. Epub 2019 Nov 12. Int J Mol Med. 2020. PMID: 31746367 Free PMC article.
References
-
- Stieber P., Heinemann V. Sinnvoller einsatz von tumormarkern/Sensible use of tumor markers. Laboratoriumsmedizin. 2008;32:339–360. doi: 10.1515/JLM.2008.015. - DOI
-
- Sturgeon C.M., Duffy M.J., Hofmann B.R., Lamerz R., Fritsche H.A., Gaarenstroom K., Bonfrer J., Ecke T.H., Grossman H.B., Hayes P., et al. National academy of clinical biochemistry laboratory medicine practice guidelines for use of tumor markers in liver, bladder, cervical, and gastric cancers. Clin. Chem. 2010;56:e1–e48. doi: 10.1373/clinchem.2009.133124. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

