Hybrid Imaging for Patient-Specific Dosimetry in Radionuclide Therapy
- PMID: 26854156
- PMCID: PMC4665601
- DOI: 10.3390/diagnostics5030296
Hybrid Imaging for Patient-Specific Dosimetry in Radionuclide Therapy
Abstract
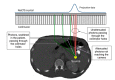
Radionuclide therapy aims to treat malignant diseases by systemic administration of radiopharmaceuticals, often using carrier molecules such as peptides and antibodies. The radionuclides used emit electrons or alpha particles as a consequence of radioactive decay, thus leading to local energy deposition. Administration to individual patients can be tailored with regards to the risk of toxicity in normal organs by using absorbed dose planning. The scintillation camera, employed in planar imaging or single-photon emission computed tomography (SPECT), generates images of the spatially and temporally varying activity distribution. Recent commercially available combined SPECT and computed tomography (CT) systems have dramatically increased the possibility of performing accurate dose planning by using the CT information in several steps of the dose-planning calculation chain. This paper discusses the dosimetry chain used for individual absorbed-dose planning and highlights the areas where hybrid imaging makes significant contributions.
Keywords: CT; Monte Carlo; SPECT; absorbed dose; activity; dosimetry; hybrid; quantitation; reconstruction; therapy.
Figures





References
-
- ICRP 2007 recommendations of the international commission on radiological protection (users edition) Ann. ICRP. 2007;37:1–332. Publication 103. - PubMed
-
- ICRP 2012 international commission on radiological protection statement on tissue reactions/early and late effects of radiation in normal tissues and organs—Threshold doses for tissue reactions in a radiation protection context. Ann. ICRP. 2012;41 doi: 10.1016/j.icrp.2012.02.001. Publication 118. - DOI - PubMed
-
- Volkert W.A., Goeckleler W.F., Ehrhardt G.J., Ketring A.R. Therapeutic radionuclides: Production and decay property considerations. J. Nucl. Med. 1991;32:174–185. - PubMed
-
- Thomadsen B E.W., Mourtada F. The physics and radiobiology of targeted radionuclide therapy. In: Speer T.W., editor. Targeted Radionuclide Therapy. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2011. pp. 71–87.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

