Neural FFA3 activation inversely regulates anion secretion evoked by nicotinic ACh receptor activation in rat proximal colon
- PMID: 26854275
- PMCID: PMC4908031
- DOI: 10.1113/JP271441
Neural FFA3 activation inversely regulates anion secretion evoked by nicotinic ACh receptor activation in rat proximal colon
Abstract
Key points: Luminal short-chain fatty acids (SCFAs) influence gut physiological function via SCFA receptors and transporters. The contribution of an SCFA receptor, free fatty acid receptor (FFA)3, to the enteric nervous system is unknown. FFA3 is expressed in enteric cholinergic neurons. Activation of neural FFA3 suppresses Cl(-) secretion induced by nicotinic ACh receptor activation via a Gi/o pathway. Neural FFA3 may have an anti-secretory function by modulating cholinergic neural reflexes in the enteric nervous system.
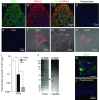
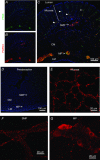
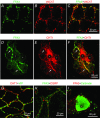
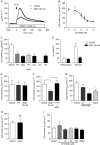
Abstract: The proximal colonic mucosa is constantly exposed to high concentrations of microbially-produced short-chain fatty acids (SCFAs). Although luminal SCFAs evoke electrogenic anion secretion and smooth muscle contractility via neural and non-neural cholinergic pathways in the colon, the involvement of the SCFA receptor free fatty acid receptor (FFA)3, one of the free fatty acid receptor family members, has not been clarified. We investigated the contribution of FFA3 to cholinergic-mediated secretory responses in rat proximal colon. FFA3 was immunolocalized to enteroendocrine cells and to the enteric neural plexuses. Most FFA3-immunoreactive nerve fibres and nerve endings were cholinergic, colocalized with protein gene product (PGP)9.5, the vesicular ACh transporter, and the high-affinity choline transporter CHT1. In Ussing chambered mucosa-submucosa preparations (including the submucosal plexus) of rat proximal colon, carbachol (CCh)-induced Cl(-) secretion was decreased by TTX, hexamethonium, and the serosal FFA3 agonists acetate or propionate, although not by an inactive analogue 3-chloropropionate. Serosal application of a selective FFA3 agonist (N-[2-methylphenyl]-[4-furan-3-yl]-2-methyl-5-oxo-1,4,5,6,7,8-hexahydro-quinoline-3-carboxamide; MQC) dose-dependently suppressed the response to CCh but not to forskolin, with an IC50 of 13 μm. Pretreatment with MQC inhibited nicotine-evoked but not bethanechol-evoked secretion. The inhibitory effect of MQC was reversed by pretreatment with pertussis toxin, indicating that FFA3 acts via the Gi/o pathway. Luminal propionate induced Cl(-) secretion via the cholinergic pathway, which was reduced by MQC, as well as by TTX, hexamethonium or removal of the submucosal plexus. These results suggest that the SCFA-FFA3 pathway has a novel anti-secretory function in that it inhibits cholinergic neural reflexes in the enteric nervous system.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures
Similar articles
-
Signaling of free fatty acid receptors 2 and 3 differs in colonic mucosa following selective agonism or coagonism by luminal propionate.Neurogastroenterol Motil. 2018 Dec;30(12):e13454. doi: 10.1111/nmo.13454. Epub 2018 Aug 23. Neurogastroenterol Motil. 2018. PMID: 30136343 Free PMC article.
-
Free fatty acid receptor 3 activation suppresses neurogenic motility in rat proximal colon.Neurogastroenterol Motil. 2018 Jan;30(1):10.1111/nmo.13157. doi: 10.1111/nmo.13157. Epub 2017 Jul 17. Neurogastroenterol Motil. 2018. PMID: 28714277 Free PMC article.
-
FFA3 Activation Stimulates Duodenal Bicarbonate Secretion and Prevents NSAID-Induced Enteropathy via the GLP-2 Pathway in Rats.Dig Dis Sci. 2017 Aug;62(8):1944-1952. doi: 10.1007/s10620-017-4600-4. Epub 2017 May 18. Dig Dis Sci. 2017. PMID: 28523577 Free PMC article.
-
Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions.J Physiol Pharmacol. 2008 Aug;59 Suppl 2:251-62. J Physiol Pharmacol. 2008. PMID: 18812643 Review.
-
Short-chain fatty acid receptor and its contribution to glucagon-like peptide-1 release.Digestion. 2014;89(1):31-6. doi: 10.1159/000356211. Epub 2014 Jan 20. Digestion. 2014. PMID: 24458110 Review.
Cited by
-
Signaling of free fatty acid receptors 2 and 3 differs in colonic mucosa following selective agonism or coagonism by luminal propionate.Neurogastroenterol Motil. 2018 Dec;30(12):e13454. doi: 10.1111/nmo.13454. Epub 2018 Aug 23. Neurogastroenterol Motil. 2018. PMID: 30136343 Free PMC article.
-
Modeling of culture conditions by culture system, glucose and propionic acid and their impact on metabolic profile in IPEC-J2.PLoS One. 2024 Jul 18;19(7):e0307411. doi: 10.1371/journal.pone.0307411. eCollection 2024. PLoS One. 2024. PMID: 39024309 Free PMC article.
-
Duodenal Chemosensing of Short-Chain Fatty Acids: Implications for GI Diseases.Curr Gastroenterol Rep. 2019 Jul 10;21(8):35. doi: 10.1007/s11894-019-0702-9. Curr Gastroenterol Rep. 2019. PMID: 31289927 Free PMC article. Review.
-
Nitric oxide in the mechanisms of inhibitory effects of sodium butyrate on colon contractions in a mouse model of irritable bowel syndrome.Naunyn Schmiedebergs Arch Pharmacol. 2025 Feb;398(2):1905-1914. doi: 10.1007/s00210-024-03403-1. Epub 2024 Aug 28. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39191960
-
Branched Short-Chain Fatty Acid Isovaleric Acid Causes Colonic Smooth Muscle Relaxation via cAMP/PKA Pathway.Dig Dis Sci. 2019 May;64(5):1171-1181. doi: 10.1007/s10620-018-5417-5. Epub 2018 Dec 17. Dig Dis Sci. 2019. PMID: 30560338 Free PMC article.
References
-
- Bellier JP & Kimura H (2007). Acetylcholine synthesis by choline acetyltransferase of a peripheral type as demonstrated in adult rat dorsal root ganglion. J Neurochem 101, 1607–1618. - PubMed
-
- Bergman EN (1990). Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev 70, 567–590. - PubMed
-
- Berthoud HR, Carlson NR & Powley TL (1991). Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol Regul Integr Comp Physiol 260, R200–R207. - PubMed
-
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A & Dowell SJ (2003). The Orphan G protein‐coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278, 11312–11319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

