Longitudinal measures of cognition in the Ts65Dn mouse: Refining windows and defining modalities for therapeutic intervention in Down syndrome
- PMID: 26854932
- PMCID: PMC5716476
- DOI: 10.1016/j.expneurol.2016.02.005
Longitudinal measures of cognition in the Ts65Dn mouse: Refining windows and defining modalities for therapeutic intervention in Down syndrome
Abstract
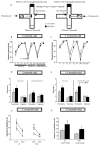
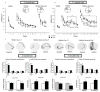
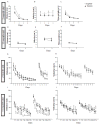
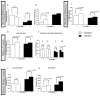
Mouse models have provided insights into adult changes in learning and memory in Down syndrome, but an in-depth assessment of how these abnormalities develop over time has never been conducted. To address this shortcoming, we conducted a longitudinal behavioral study from birth until late adulthood in the Ts65Dn mouse model to measure the emergence and continuity of learning and memory deficits in individuals with a broad array of tests. Our results demonstrate for the first time that the pace at which neonatal and perinatal milestones are acquired is correlated with later cognitive performance as an adult. In addition, we find that life-long behavioral indexing stratifies mice within each genotype. Our expanded assessment reveals that diminished cognitive flexibility, as measured by reversal learning, is the most robust learning and memory impairment in both young and old Ts65Dn mice. Moreover, we find that reversal learning degrades with age and is therefore a useful biomarker for studying age-related decline in cognitive ability. Altogether, our results indicate that preclinical studies aiming to restore cognitive function in Ts65Dn should target both neonatal milestones and reversal learning in adulthood. Here we provide the quantitative framework for this type of approach.
Keywords: Aging; Alzheimer Disease; Cognition; Developmental disorder; Developmental milestones; Down syndrome; Mouse behavior; Mouse model; Reversal learning; Ts65Dn.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Low dose EGCG treatment beginning in adolescence does not improve cognitive impairment in a Down syndrome mouse model.Pharmacol Biochem Behav. 2015 Nov;138:70-9. doi: 10.1016/j.pbb.2015.09.002. Epub 2015 Sep 10. Pharmacol Biochem Behav. 2015. PMID: 26363314
-
Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down's syndrome.Exp Neurol. 2000 Feb;161(2):647-63. doi: 10.1006/exnr.1999.7289. Exp Neurol. 2000. PMID: 10686084
-
Effects of chronic administration of SGS-111 during adulthood and during the pre- and post-natal periods on the cognitive deficits of Ts65Dn mice, a model of Down syndrome.Behav Brain Res. 2008 Apr 9;188(2):355-67. doi: 10.1016/j.bbr.2007.11.020. Epub 2007 Dec 3. Behav Brain Res. 2008. PMID: 18178265
-
State-of-the-art therapy for Down syndrome.Dev Med Child Neurol. 2023 Jul;65(7):870-884. doi: 10.1111/dmcn.15517. Epub 2023 Jan 24. Dev Med Child Neurol. 2023. PMID: 36692980 Review.
-
Neurobehavioral biomarkers of aging: influence of genotype and dietary restriction.Biomed Environ Sci. 1991 Jun;4(1-2):144-65. Biomed Environ Sci. 1991. PMID: 1910592 Review.
Cited by
-
Enhanced GIRK2 channel signaling in Down syndrome: A feasible role in the development of abnormal nascent neural circuits.Front Genet. 2022 Sep 12;13:1006068. doi: 10.3389/fgene.2022.1006068. eCollection 2022. Front Genet. 2022. PMID: 36171878 Free PMC article. Review.
-
Longitudinal neuroanatomical and behavioral analyses show phenotypic drift and variability in the Ts65Dn mouse model of Down syndrome.Dis Model Mech. 2020 Sep 25;13(9):dmm046243. doi: 10.1242/dmm.046243. Dis Model Mech. 2020. PMID: 32817053 Free PMC article.
-
Time-dependent diffusion MRI probes cerebellar microstructural alterations in a mouse model of Down syndrome.Brain Commun. 2021 Apr 5;3(2):fcab062. doi: 10.1093/braincomms/fcab062. eCollection 2021. Brain Commun. 2021. PMID: 33937769 Free PMC article.
-
Spaced training improves learning in Ts65Dn and Ube3a mouse models of intellectual disabilities.Transl Psychiatry. 2019 Jun 10;9(1):166. doi: 10.1038/s41398-019-0495-5. Transl Psychiatry. 2019. PMID: 31182707 Free PMC article.
-
Early neurotrophic pharmacotherapy rescues developmental delay and Alzheimer's-like memory deficits in the Ts65Dn mouse model of Down syndrome.Sci Rep. 2017 Apr 3;7:45561. doi: 10.1038/srep45561. Sci Rep. 2017. PMID: 28368015 Free PMC article.
References
-
- Altafaj X, Martin ED, Ortiz-Abalia J, Valderrama A, Lao-Peregrin C, Dierssen M, Fillat C. Normalization of Dyrk1A expression by AAV2/1-shDyrk1A attenuates hippocampal-dependent defects in the Ts65Dn mouse model of Down syndrome. Neurobiol Dis. 2013;52:117–127. - PubMed
-
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychology review. 2006;16:17–42. - PubMed
-
- Breslin J, Spano G, Bootzin R, Anand P, Nadel L, Edgin J. Obstructive sleep apnea syndrome and cognition in Down syndrome. Developmental medicine and child neurology. 2014;56:657–664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

