Biliary fibrosis drives liver repopulation and phenotype transition of transplanted hepatocytes
- PMID: 26855174
- PMCID: PMC5137249
- DOI: 10.1016/j.jhep.2016.01.036
Biliary fibrosis drives liver repopulation and phenotype transition of transplanted hepatocytes
Abstract
Background & aims: Current research focuses on developing alternative strategies to restore decreased liver mass prior to the onset of end-stage liver disease. Cell engraftment/repopulation requires regeneration in normal liver, but we have shown that severe liver injury stimulates repopulation without partial hepatectomy (PH). We have now investigated whether a less severe injury, secondary biliary fibrosis, would drive engraftment/repopulation of ectopically transplanted mature hepatocytes.
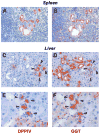
Methods: Ductular proliferation and progressive fibrosis in dipeptidyl-peptidase IV (DPPIV)(-) F344 rats was induced by common bile duct ligation (BDL). Purified DPPIV(+)/green fluorescent protein (GFP)(+) hepatocytes were infused without PH into the spleen of BDL rats and compared to rats without BDL.
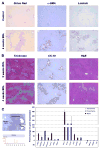
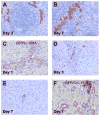
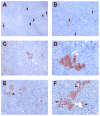
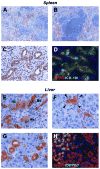
Results: Within one week, transplanted hepatocytes were detected in hepatic portal areas and at the periphery of expanding portal regions. DPPIV(+)/GFP(+) repopulating cell clusters of different sizes were observed in BDL rats but not untreated normal recipients. Surprisingly, some engrafted hepatocytes formed CK-19/claudin-7 expressing epithelial cells resembling cholangiocytes within repopulating clusters. In addition, substantial numbers of hepatocytes engrafted at the intrasplenic injection site assembled into multicellular groups. These also showed biliary "transdifferentiation" in the majority of intrasplenic injection sites of rats that received BDL but not in untreated recipients. PCR array analysis showed upregulation of osteopontin (SPP1). Cell culture studies demonstrated increased Itgβ4, HNF1β, HNF6, Sox-9, and CK-19 mRNA expression in hepatocytes incubated with osteopontin, suggesting that this secreted protein promotes dedifferentiation of hepatocytes.
Conclusions: Our studies show that biliary fibrosis stimulates liver repopulation by ectopically transplanted hepatocytes and also stimulates hepatocyte transition towards a biliary epithelial phenotype.
Keywords: Bile duct ligation; Cell transplantation; Hepatocyte dedifferentiation; Hepatocyte transdifferentiation; Osteopontin.
Copyright © 2016 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures


Similar articles
-
Contribution of Mature Hepatocytes to Biliary Regeneration in Rats with Acute and Chronic Biliary Injury.PLoS One. 2015 Aug 26;10(8):e0134327. doi: 10.1371/journal.pone.0134327. eCollection 2015. PLoS One. 2015. PMID: 26308208 Free PMC article.
-
Stem cell properties and repopulation of the rat liver by fetal liver epithelial progenitor cells.Am J Pathol. 2001 Oct;159(4):1323-34. doi: 10.1016/S0002-9440(10)62519-9. Am J Pathol. 2001. PMID: 11583960 Free PMC article.
-
Transdifferentiation into biliary ductular cells of hepatocytes transplanted into the spleen.Pathology. 2008 Apr;40(3):272-6. doi: 10.1080/00313020801911546. Pathology. 2008. PMID: 18428047
-
Aberrant differentiation and proliferation of hepatocytes in chronic liver injury and liver tumors.Pathol Int. 2024 Jul;74(7):361-378. doi: 10.1111/pin.13441. Epub 2024 Jun 5. Pathol Int. 2024. PMID: 38837539 Free PMC article. Review.
-
Liver irradiation: a potential preparative regimen for hepatocyte transplantation.Int J Radiat Oncol Biol Phys. 2001 Feb 1;49(2):451-7. doi: 10.1016/s0360-3016(00)01495-4. Int J Radiat Oncol Biol Phys. 2001. PMID: 11173140 Review.
Cited by
-
Autophagy suppresses the formation of hepatocyte-derived cancer-initiating ductular progenitor cells in the liver.Sci Adv. 2021 Jun 4;7(23):eabf9141. doi: 10.1126/sciadv.abf9141. Print 2021 Jun. Sci Adv. 2021. PMID: 34088666 Free PMC article.
-
Biological and clinical insights offered by chemically induced liver progenitors (CLiPs).Stem Cell Investig. 2017 Aug 21;4:68. doi: 10.21037/sci.2017.08.04. eCollection 2017. Stem Cell Investig. 2017. PMID: 28920061 Free PMC article. No abstract available.
-
Wnt signaling regulates hepatobiliary repair following cholestatic liver injury in mice.Hepatology. 2016 Nov;64(5):1652-1666. doi: 10.1002/hep.28774. Epub 2016 Sep 26. Hepatology. 2016. PMID: 27533619 Free PMC article.
-
Transcriptomic Dissection of Hepatocyte Heterogeneity: Linking Ploidy, Zonation, and Stem/Progenitor Cell Characteristics.Cell Mol Gastroenterol Hepatol. 2020;9(1):161-183. doi: 10.1016/j.jcmgh.2019.08.011. Epub 2019 Sep 5. Cell Mol Gastroenterol Hepatol. 2020. PMID: 31493546 Free PMC article.
-
Role of Immune Cells in Biliary Repair.Front Immunol. 2022 Mar 30;13:866040. doi: 10.3389/fimmu.2022.866040. eCollection 2022. Front Immunol. 2022. PMID: 35432349 Free PMC article. Review.
References
-
- Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. - PubMed
-
- Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61:1–117. - PubMed
-
- Cárdenas A, Ginès P. Management of patients with cirrhosis awaiting liver transplantation. Gut. 2011;60:412–421. - PubMed
-
- Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, et al. OPTN/SRTR 2013 Annual Data Report: liver. Am J Transplant. 2015;15(Suppl 2):1–28. - PubMed
-
- Struecker B, Raschzok N, Sauer IM. Liver support strategies: cutting-edge technologies. Nat Rev Gastroenterol Hepatol. 2014;11:166–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

