Response to Therapy in Antiretroviral Therapy-Naive Patients With Isolated Nonnucleoside Reverse Transcriptase Inhibitor-Associated Transmitted Drug Resistance
- PMID: 26855248
- PMCID: PMC4866916
- DOI: 10.1097/QAI.0000000000000942
Response to Therapy in Antiretroviral Therapy-Naive Patients With Isolated Nonnucleoside Reverse Transcriptase Inhibitor-Associated Transmitted Drug Resistance
Abstract
Background: Nonnucleoside reverse transcriptase inhibitor (NNRTI)-associated transmitted drug resistance (TDR) is the most common type of TDR. Few data guide the selection of antiretroviral therapy (ART) for patients with such resistance.
Methods: We reviewed treatment outcomes in a cohort of HIV-1-infected patients with isolated NNRTI TDR who initiated ART between April 2002 and May 2014. In an as-treated analysis, virological failure (VF) was defined as not reaching undetectable virus levels within 24 weeks, virological rebound, or switching regimens during viremia. In an intention-to-treat analysis, failure was defined more broadly as VF, loss to follow-up, and switching during virological suppression.
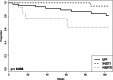
Results: Of 3245 patients, 131 (4.0%) had isolated NNRTI TDR; 122 received a standard regimen comprising 2 NRTIs plus a boosted protease inhibitor (bPI; n = 54), an integrase strand transfer inhibitor (INSTI; n = 52), or an NNRTI (n = 16). The median follow-up was 100 weeks. In the as-treated analysis, VF occurred in 15% (n = 8), 2% (n = 1), and 25% (n = 4) of patients in the bPI, INSTI, and NNRTI groups, respectively. In multivariate regression, there was a trend toward a lower risk of VF with INSTIs than with bPIs (hazard ratio: 0.14; 95% confidence interval: 0.02 to 1.1; P = 0.07). In intention-to-treat multivariate regression, INSTIs had a lower risk of failure than bPIs (hazard ratio: 0.38; 95% confidence interval: 0.18 to 0.82; P = 0.01).
Conclusions: Patients with isolated NNRTI TDR experienced low VF rates with INSTIs and bPIs. INSTIs were noninferior to bPIs in an analysis of VF but superior to bPIs when frequency of switching and loss to follow-up were also considered.
Conflict of interest statement
D.S.C. was supported by a grant for this work (T32 AI052073) from the National Institutes of Health. S.-Y.R. and R.W.S. were also supported by a grant (R01 AI068581) from the National Institutes of Health. D.S.C. to receive research funding through the Bristol-Myers Squibb Virology Fellows Research Program in March 2016. R.W.S. is a consultant for Celera, and receives research funding from Roche Molecular, Gilead Sciences, Bristol-Myers Squibb, and Merck. The other authors have no funding or conflicts of interest to disclose.
Figures
Similar articles
-
Impact of transmitted HIV-1 drug resistance on the efficacy of first-line antiretroviral therapy with two nucleos(t)ide reverse transcriptase inhibitors plus an integrase inhibitor or a protease inhibitor.J Antimicrob Chemother. 2018 Sep 1;73(9):2480-2484. doi: 10.1093/jac/dky211. J Antimicrob Chemother. 2018. PMID: 29945251
-
Resistance at virological failure using boosted protease inhibitors versus nonnucleoside reverse transcriptase inhibitors as first-line antiretroviral therapy--implications for sustained efficacy of ART in resource-limited settings.J Infect Dis. 2013 Jun 15;207 Suppl 2:S78-84. doi: 10.1093/infdis/jit112. J Infect Dis. 2013. PMID: 23687293
-
Predictors of low-level HIV viraemia and virological failure in the era of integrase inhibitors: A Spanish nationwide cohort.HIV Med. 2022 Sep;23(8):825-836. doi: 10.1111/hiv.13265. Epub 2022 Mar 1. HIV Med. 2022. PMID: 35234328
-
Emergence of drug resistance in HIV type 1-infected patients after receipt of first-line highly active antiretroviral therapy: a systematic review of clinical trials.Clin Infect Dis. 2008 Sep 1;47(5):712-22. doi: 10.1086/590943. Clin Infect Dis. 2008. PMID: 18662137
-
Switching regimens in virologically suppressed HIV-1-infected patients: evidence base and rationale for integrase strand transfer inhibitor (INSTI)-containing regimens.HIV Med. 2016 Oct;17 Suppl 5:3-16. doi: 10.1111/hiv.12440. HIV Med. 2016. PMID: 27714978 Review.
Cited by
-
Prevalence of Drug-Resistant Minority Variants in Untreated HIV-1-Infected Individuals With and Those Without Transmitted Drug Resistance Detected by Sanger Sequencing.J Infect Dis. 2017 Aug 1;216(3):387-391. doi: 10.1093/infdis/jix338. J Infect Dis. 2017. PMID: 28859436 Free PMC article.
-
HIV-1 drug resistance and resistance testing.Infect Genet Evol. 2016 Dec;46:292-307. doi: 10.1016/j.meegid.2016.08.031. Epub 2016 Aug 29. Infect Genet Evol. 2016. PMID: 27587334 Free PMC article. Review.
-
Risk of HIV viral rebound in HIV infected patients on direct acting antivirals (DAAs) treatment for HCV.PLoS One. 2022 Feb 3;17(2):e0262917. doi: 10.1371/journal.pone.0262917. eCollection 2022. PLoS One. 2022. PMID: 35113890 Free PMC article.
-
The World Health Organization's Response to Emerging Human Immunodeficiency Virus Drug Resistance and a Call for Global Action.J Infect Dis. 2017 Dec 1;216(suppl_9):S801-S804. doi: 10.1093/infdis/jix402. J Infect Dis. 2017. PMID: 29040686 Free PMC article.
-
Clinical Impact of Pretreatment Human Immunodeficiency Virus Drug Resistance in People Initiating Nonnucleoside Reverse Transcriptase Inhibitor-Containing Antiretroviral Therapy: A Systematic Review and Meta-analysis.J Infect Dis. 2021 Aug 2;224(3):377-388. doi: 10.1093/infdis/jiaa683. J Infect Dis. 2021. PMID: 33202025 Free PMC article.
References
-
- Wheeler WH, Ziebell RA, Zabina H, et al. Prevalence of transmitted drug resistance associated mutations and HIV-1 subtypes in new HIV-1 diagnoses, U.S.-2006. AIDS. 2010;24:1203–1212. - PubMed
-
- Li J, Kim D, Linley L, et al. Sensitive screening reveals widesperad underestimation of transmitted HIV drug resistance [87]. Presented at: Conference on Retroviruses and Opportunistic Infections; March 3–6, 2014; Boston, MA.
-
- Ocfemia MCB. Epidemiology of HIV-1 transmitted drug resistance among men who have sex with men in the United States [81]. Presented at: XXIV International HIV Drug Resistance Workshop; February 21–22, 2015; Seattle, WA.
-
- Oette M, Kaiser R, Daumer M, et al. Primary HIV drug resistance and efficacy of first-line antiretroviral therapy guided by resistance testing. J Acquir Immune Defic Syndr. 2006;41:573–581. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

