Complementary subicular pathways to the anterior thalamic nuclei and mammillary bodies in the rat and macaque monkey brain
- PMID: 26855336
- PMCID: PMC4855639
- DOI: 10.1111/ejn.13208
Complementary subicular pathways to the anterior thalamic nuclei and mammillary bodies in the rat and macaque monkey brain
Abstract
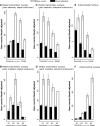
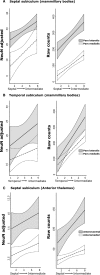
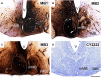
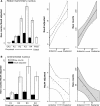
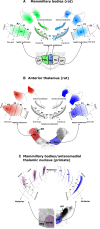
The origins of the hippocampal (subicular) projections to the anterior thalamic nuclei and mammillary bodies were compared in rats and macaque monkeys using retrograde tracers. These projections form core components of the Papez circuit, which is vital for normal memory. The study revealed a complex pattern of subicular efferents, consistent with the presence of different, parallel information streams, whose segregation appears more marked in the rat brain. In both species, the cells projecting to the mammillary bodies and anterior thalamic nuclei showed laminar separation but also differed along other hippocampal axes. In the rat, these diencephalic inputs showed complementary topographies in the proximal-distal (columnar) plane, consistent with differential involvement in object-based (proximal subiculum) and context-based (distal subiculum) information. The medial mammillary inputs, which arose along the anterior-posterior extent of the rat subiculum, favoured the central subiculum (septal hippocampus) and the more proximal subiculum (temporal hippocampus). In contrast, anterior thalamic inputs were largely confined to the dorsal (i.e. septal and intermediate) subiculum, where projections to the anteromedial nucleus favoured the proximal subiculum while those to the anteroventral nucleus predominantly arose in the distal subiculum. In the macaque, the corresponding diencephalic inputs were again distinguished by anterior-posterior topographies, as subicular inputs to the medial mammillary bodies predominantly arose from the posterior hippocampus while subicular inputs to the anteromedial thalamic nucleus predominantly arose from the anterior hippocampus. Unlike the rat, there was no clear evidence of proximal-distal separation as all of these medial diencephalic projections preferentially arose from the more distal subiculum.
Keywords: anatomy; fornix; hippocampus; memory; subiculum.
© 2016 The Authors. European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures





Similar articles
-
Parallel but separate inputs from limbic cortices to the mammillary bodies and anterior thalamic nuclei in the rat.J Comp Neurol. 2010 Jun 15;518(12):2334-54. doi: 10.1002/cne.22336. J Comp Neurol. 2010. PMID: 20437531 Free PMC article.
-
Projections from the hippocampal region to the mammillary bodies in macaque monkeys.Eur J Neurosci. 2005 Nov;22(10):2519-30. doi: 10.1111/j.1460-9568.2005.04450.x. Eur J Neurosci. 2005. PMID: 16307594
-
Monosynaptic connection from the subiculum to medial mammillary nucleus neurons projecting to the anterior thalamus and Gudden's ventral tegmental nucleus.Neurosci Res. 2021 Oct;171:1-8. doi: 10.1016/j.neures.2021.01.006. Epub 2021 Jan 18. Neurosci Res. 2021. PMID: 33476683
-
How do mammillary body inputs contribute to anterior thalamic function?Neurosci Biobehav Rev. 2015 Jul;54:108-19. doi: 10.1016/j.neubiorev.2014.07.025. Epub 2014 Aug 11. Neurosci Biobehav Rev. 2015. PMID: 25107491 Free PMC article. Review.
-
Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions.Eur J Neurosci. 2010 Jun;31(12):2292-307. doi: 10.1111/j.1460-9568.2010.07251.x. Epub 2010 Jun 14. Eur J Neurosci. 2010. PMID: 20550571 Free PMC article. Review.
Cited by
-
Hippocampus: Molecular, Cellular, and Circuit Features in Anxiety.Neurosci Bull. 2023 Jun;39(6):1009-1026. doi: 10.1007/s12264-023-01020-1. Epub 2023 Jan 21. Neurosci Bull. 2023. PMID: 36680709 Free PMC article. Review.
-
Alteration of Neural Pathways and Its Implications in Alzheimer's Disease.Biomedicines. 2022 Apr 4;10(4):845. doi: 10.3390/biomedicines10040845. Biomedicines. 2022. PMID: 35453595 Free PMC article. Review.
-
The anterior thalamic nuclei and cognition: A role beyond space?Neurosci Biobehav Rev. 2021 Jul;126:1-11. doi: 10.1016/j.neubiorev.2021.02.047. Epub 2021 Mar 15. Neurosci Biobehav Rev. 2021. PMID: 33737105 Free PMC article. Review.
-
The anterior thalamic nuclei and nucleus reuniens: So similar but so different.Neurosci Biobehav Rev. 2020 Dec;119:268-280. doi: 10.1016/j.neubiorev.2020.10.006. Epub 2020 Oct 16. Neurosci Biobehav Rev. 2020. PMID: 33069688 Free PMC article. Review.
-
Deconstructing the Direct Reciprocal Hippocampal-Anterior Thalamic Pathways for Spatial Learning.J Neurosci. 2020 Sep 2;40(36):6978-6990. doi: 10.1523/JNEUROSCI.0874-20.2020. Epub 2020 Aug 4. J Neurosci. 2020. PMID: 32753513 Free PMC article.
References
-
- Aggleton, J.P. (1985) X‐ray localization of limbic structures in the cynomolgus monkey (Macaca fascicularis). J. Neurosci. Meth., 14, 101–108. - PubMed
-
- Aggleton, J.P. (1986) A description of the amygdalo‐hippocampal interconnections in the macaque monkey. Exp. Brain Res., 64, 515–526. - PubMed
-
- Aggleton, J.P. (2012) Multiple anatomical systems embedded within the primate medial temporal lobe: implications for hippocampal function. Neurosci. Biobehav. R., 36, 1579–1596. - PubMed
-
- Aggleton, J.P. & Brown, M.W. (1999) Episodic memory, amnesia, and the hippocampal‐anterior thalamic axis. Behav. Brain Sci., 22, 425–444. - PubMed
-
- Aggleton, J.P. & Christiansen, K. (2015) The subiculum: the heart of the extended hippocampal system. Prog. Brain Res., 219, 65–82. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

