Chemical and biological mechanisms of phytochemical activation of Nrf2 and importance in disease prevention
- PMID: 26855455
- PMCID: PMC4739799
- DOI: 10.1007/978-3-319-00581-2_7
Chemical and biological mechanisms of phytochemical activation of Nrf2 and importance in disease prevention
Abstract
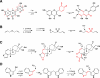
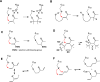
Plants are an incredibly rich source of compounds that activate the Nrf2 transcription factor, leading to upregulation of a battery of cytoprotective genes. This perspective surveys established and proposed molecular mechanisms of Nrf2 activation by phytochemicals with a special emphasis on a common chemical property of Nrf2 activators: the ability as "soft" electrophiles to modify cellular thiols, either directly or as oxidized biotransformants. In addition, the role of reactive oxygen/nitrogen species as secondary messengers in Nrf2 activation is discussed. While the uniquely reactive C151 of Keap1, an Nrf2 repressor protein, is highlighted as a key target of cytoprotective phytochemicals, also reviewed are other stress-responsive proteins, including kinases, which play non-redundant roles in the activation of Nrf2 by plant-derived agents. Finally, the perspective presents two key factors accounting for the enhanced therapeutic windows of effective phytochemical activators of the Keap1-Nrf2 axis: enhanced selectivity toward sensor cysteines and reversibility of addition to thiolate molecules.
Figures



Similar articles
-
Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals.Planta Med. 2008 Oct;74(13):1526-39. doi: 10.1055/s-0028-1088302. Epub 2008 Oct 20. Planta Med. 2008. PMID: 18937164 Review.
-
Targeting Nrf2-Keap1 signaling for chemoprevention of skin carcinogenesis with bioactive phytochemicals.Toxicol Lett. 2014 Aug 17;229(1):73-84. doi: 10.1016/j.toxlet.2014.05.018. Epub 2014 May 27. Toxicol Lett. 2014. PMID: 24875534 Review.
-
Natural dietary anti-cancer chemopreventive compounds: redox-mediated differential signaling mechanisms in cytoprotection of normal cells versus cytotoxicity in tumor cells.Acta Pharmacol Sin. 2007 Apr;28(4):459-72. doi: 10.1111/j.1745-7254.2007.00549.x. Acta Pharmacol Sin. 2007. PMID: 17376285 Review.
-
Nrf2: friend and foe in preventing cigarette smoking-dependent lung disease.Chem Res Toxicol. 2012 Sep 17;25(9):1805-24. doi: 10.1021/tx300145n. Epub 2012 Jun 22. Chem Res Toxicol. 2012. PMID: 22686525 Review.
-
Succinylation of a KEAP1 sensor lysine promotes NRF2 activation.bioRxiv [Preprint]. 2023 May 9:2023.05.08.539908. doi: 10.1101/2023.05.08.539908. bioRxiv. 2023. Update in: Cell Chem Biol. 2023 Oct 19;30(10):1295-1302.e4. doi: 10.1016/j.chembiol.2023.07.014. PMID: 37215033 Free PMC article. Updated. Preprint.
Cited by
-
On the Role of ROS and Glutathione in the Mode of Action Underlying Nrf2 Activation by the Hydroxyanthraquinone Purpurin.Antioxidants (Basel). 2023 Aug 2;12(8):1544. doi: 10.3390/antiox12081544. Antioxidants (Basel). 2023. PMID: 37627539 Free PMC article.
-
Exploitation of Agro-Industrial Waste as Potential Source of Bioactive Compounds for Aquaculture.Foods. 2020 Jun 28;9(7):843. doi: 10.3390/foods9070843. Foods. 2020. PMID: 32605275 Free PMC article. Review.
-
Healthy Diets and Lifestyles in the World: Mediterranean and Blue Zone People Live Longer. Special Focus on Gut Microbiota and Some Food Components.Endocr Metab Immune Disord Drug Targets. 2024;24(15):1774-1784. doi: 10.2174/0118715303271634240319054728. Endocr Metab Immune Disord Drug Targets. 2024. PMID: 38566378 Review.
-
Mitochondria-Centric Review of Polyphenol Bioactivity in Cancer Models.Antioxid Redox Signal. 2018 Dec 1;29(16):1589-1611. doi: 10.1089/ars.2017.7404. Epub 2017 Dec 11. Antioxid Redox Signal. 2018. PMID: 29084444 Free PMC article. Review.
-
Impact of Polyphenolic-Food on Longevity: An Elixir of Life. An Overview.Antioxidants (Basel). 2021 Mar 24;10(4):507. doi: 10.3390/antiox10040507. Antioxidants (Basel). 2021. PMID: 33805092 Free PMC article. Review.
References
-
- Kasai H, Kawai K. Oxidative DNA damage: mechanisms and significance in health and disease. Antioxid Redox Signal. 2006;8:981–983. - PubMed
-
- Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C, Shyr C, Wakabayashi N, Kensler TW, Wasserman WW, Biswal S. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 2010;38:5718–5734. - PMC - PubMed
-
- Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–188. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
