Hemoglobin levels and quality of life in patients with symptomatic chemotherapy-induced anemia: the eAQUA study
- PMID: 26855598
- PMCID: PMC4725626
- DOI: 10.2147/CMAR.S88110
Hemoglobin levels and quality of life in patients with symptomatic chemotherapy-induced anemia: the eAQUA study
Abstract
Purpose: To assess hemoglobin (Hb) outcomes and fatigue-related quality-of-life (QoL) (electronic assessment) in patients with solid tumors and symptomatic chemotherapy-induced anemia receiving cytotoxic chemotherapy and darbepoetin alfa (DA) or another erythropoiesis-stimulating agent according to European indication.
Methods: eAQUA was a Phase IV prospective observational study. The primary outcome (assessed in the primary analysis set [PAS]: patients receiving one or more DA dose who had baseline and week 9 assessments for Hb and QoL) was the proportion of patients receiving DA having both Hb increases ≥1 g/dL and improved QoL between baseline and week 9. Functional Assessment of Cancer Therapy-Fatigue (FACT-F) subscale scores were anchored to fatigue visual analog scale scores to determine the minimally important difference for improved QoL. Overall data/data over time are reported for the full analysis set (patients receiving one or more erythropoiesis-stimulating agent dose, n=1,158); week 9 data (ie, data relating to the primary and secondary outcomes) are reported for the PAS (n=510). Baseline and safety data are included for both the full analysis set and PAS.
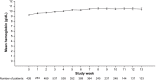
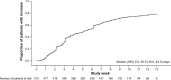
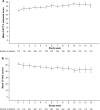
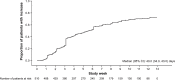
Results: In the PAS, 69% of patients had stage IV disease and 96% were fatigued. The minimally important difference in FACT-F change score for QoL improvement was 3.5. From baseline to week 9, 32% (95% confidence interval: 28%-36%) of patients had both improved QoL and an Hb increase ≥1 g/dL; proportions were similar across the most common tumor types. At week 9, 49% and 58% of patients had improved QoL or Hb increases ≥1 g/dL, respectively; 70% and 76% had QoL or Hb improvements between baseline and study end, respectively. In the PAS, 16% of patients required transfusions and 32% required iron supplementation. Few patients (<1%) reported adverse drug reactions.
Conclusion: In this study, patients with solid tumors receiving DA per European indication for symptomatic chemotherapy-induced anemia had clinically meaningful improvements in Hb and QoL.
Keywords: Functional Assessment of Cancer Therapy-Fatigue subscale; darbepoetin alfa; erythropoiesis-stimulating agent; fatigue visual analog scale; transfusion.
Figures
References
-
- Ahlberg K, Ekman T, Gaston-Johansson F, Mock V. Assessment and management of cancer-related fatigue in adults. Lancet. 2003;362(9384):640–650. - PubMed
-
- Harper P, Littlewood T. Anaemia of cancer: impact on patient fatigue and long-term outcome. Oncology. 2005;69(Suppl 2):2–7. - PubMed
-
- Weis J. Cancer-related fatigue: prevalence, assessment and treatment strategies. Expert Rev Pharmacoecon Outcomes Res. 2011;11(4):441–446. - PubMed
-
- Campos MP, Hassan BJ, Riechelmann R, Del Giglio A. Cancer-related fatigue: a review. Rev Assoc Med Bras. 2011;57(2):211–219. English, Portuguese. - PubMed
-
- Iop A, Manfredi AM, Bonura S. Fatigue in cancer patients receiving chemotherapy: an analysis of published studies. Ann Oncol. 2004;15(5):712–720. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

