Change in Chirality of Semiconducting Single-Walled Carbon Nanotubes Can Overcome Anionic Surfactant Stabilization: A Systematic Study of Aggregation Kinetics
- PMID: 26855611
- PMCID: PMC4742347
- DOI: 10.1071/EN14176
Change in Chirality of Semiconducting Single-Walled Carbon Nanotubes Can Overcome Anionic Surfactant Stabilization: A Systematic Study of Aggregation Kinetics
Abstract
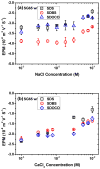
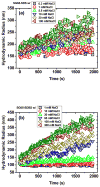
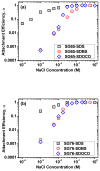
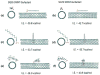
Single-walled carbon nanotubes' (SWNT) effectiveness in applications is enhanced by debundling or stabilization. Anionic surfactants are known to effectively stabilize SWNTs. However, the role of specific chirality on surfactant-stabilized SWNT aggregation has not been studied to date. The aggregation behavior of chirally enriched (6,5) and (7,6) semiconducting SWNTs, functionalized with three anionic surfactants-sodium dodecyl sulfate (SDS), sodium dodecyl benzene sulfonate (SDBS), and sodium deoxycholate (SDOCO)-was evaluated with time-resolved dynamic light scattering. A wide range of mono- (NaCl) and di-valent (CaCl2) electrolytes as well as a 2.5 mg TOC/L Suwannee River humic acid (SRHA) were used as background chemistry. Overall, SDBS showed the most effectiveness in SWNT stability, followed by SDOCO and SDS. However, the relatively larger diameter (7,6) chiral tubes compromised the surfactant stability, compared to (6,5) chiral enrichment, due to enhanced van der Waals interaction. The presence of di-valent electrolytes overshadowed the chirality effects and resulted in similar aggregation behavior for both the SWNT samples. Molecular modeling results enumerated key differences in surfactant conformation on SWNT surfaces and identified interaction energy changes between the two chiralities to delineate aggregation mechanisms. The stability of SWNTs increased in the presence of SRHA under 10 mM monovalent and mixed electrolyte conditions. The results suggest that change in chirality can overcome surfactant stabilization of semiconducting SWNTs. SWNT stability can also be strongly influenced by the anionic surfactant structure.
Keywords: Chirality; aggregation kinetics; anionic surfactants; molecular dynamic simulation; single-walled carbon nanotube; stability.
Figures



Similar articles
-
Chirality affects aggregation kinetics of single-walled carbon nanotubes.Environ Sci Technol. 2013 Feb 19;47(4):1844-52. doi: 10.1021/es3030337. Epub 2013 Feb 5. Environ Sci Technol. 2013. PMID: 23343128 Free PMC article.
-
Quantitative theory of adsorptive separation for the electronic sorting of single-walled carbon nanotubes.ACS Nano. 2014 Apr 22;8(4):3367-79. doi: 10.1021/nn4058402. Epub 2014 Mar 12. ACS Nano. 2014. PMID: 24606316
-
Tuning the Surface Ordering of Self-Assembled Ionic Surfactants on Semiconducting Single-Walled Carbon Nanotubes: Concentration, Tube Diameter, and Counterions.Langmuir. 2018 Aug 7;34(31):9279-9288. doi: 10.1021/acs.langmuir.8b01813. Epub 2018 Jul 25. Langmuir. 2018. PMID: 30008207
-
Gel Chromatography for Separation of Single-Walled Carbon Nanotubes.Gels. 2022 Jan 24;8(2):76. doi: 10.3390/gels8020076. Gels. 2022. PMID: 35200458 Free PMC article. Review.
-
Designing Catalysts for Chirality-Selective Synthesis of Single-Walled Carbon Nanotubes: Past Success and Future Opportunity.Adv Mater. 2019 Mar;31(9):e1800805. doi: 10.1002/adma.201800805. Epub 2018 Aug 30. Adv Mater. 2019. PMID: 30160811 Review.
Cited by
-
Examination of Single-Walled Carbon Nanotubes Uptake and Toxicity from Dietary Exposure: Tracking Movement and Impacts in the Gastrointestinal System.Nanomaterials (Basel). 2015 Jun 12;5(2):1066-1086. doi: 10.3390/nano5021066. Nanomaterials (Basel). 2015. PMID: 28347052 Free PMC article.
-
Hydroxyl functionalized multi-walled carbon nanotubes modulate immune responses without increasing 2009 pandemic influenza A/H1N1 virus titers in infected mice.Toxicol Appl Pharmacol. 2020 Oct 1;404:115167. doi: 10.1016/j.taap.2020.115167. Epub 2020 Aug 7. Toxicol Appl Pharmacol. 2020. PMID: 32771490 Free PMC article.
-
Insights into Metal Oxide and Zero-Valent Metal Nanocrystal Formation on Multiwalled Carbon Nanotube Surfaces during Sol-Gel Process.Nanomaterials (Basel). 2018 Jun 5;8(6):403. doi: 10.3390/nano8060403. Nanomaterials (Basel). 2018. PMID: 29874789 Free PMC article.
-
Aggregation Behavior of Multiwalled Carbon Nanotube-Titanium Dioxide Nanohybrids: Probing the Part-Whole Question.Environ Sci Technol. 2018 Aug 7;52(15):8233-8241. doi: 10.1021/acs.est.7b05826. Epub 2018 Jul 10. Environ Sci Technol. 2018. PMID: 29944362 Free PMC article.
References
-
- Iijima S, Ichihashi T. Single-Shell Carbon Nanotubes of 1-nm Diameter. Nature. 1993;363:603–5.
-
- Saito R, Dresselhaus G, Dresselhaus MS. Physical properties of carbon nanotubes. Imperial college press; London: 1998.
-
- Weisman RB. In: Contemporary Concepts of Condensed Matter Science. Saito S, Zettl A, editors. Elsevier; 2008. pp. 109–33.
-
- Baughman RH, Zakhidov AA, de Heer WA. Carbon nanotubes - the route toward applications. Science. 2002;297(5582):787–92. - PubMed
-
- Chen KJ, Nair N, Strano MS, Braatz RD. Identification of chirality-dependent adsorption kinetics in single-walled carbon nanotube reaction networks. J Comput Theor Nanosci. 2010;7(12):2581–5.
Grants and funding
LinkOut - more resources
Full Text Sources
