Alternatives to the 'water oxidation pathway' of biological ozone formation
- PMID: 26855676
- PMCID: PMC4733074
- DOI: 10.1007/s12154-015-0140-6
Alternatives to the 'water oxidation pathway' of biological ozone formation
Abstract
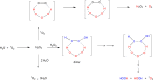
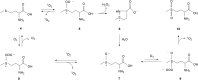
Recent studies have shown that ozone (O3) is endogenously generated in living tissues, where it makes both positive and negative physiological contributions. A pathway for the formation of both O3 and hydrogen peroxide (H2O2) was previously proposed, beginning with the antibody or amino acid-catalyzed oxidation of water by singlet oxygen ((1)O2) to form hydrogen trioxide (H2O3) as a key intermediate. A key pillar of this hypothesis is that some of the H2O2 molecules incorporate water-derived oxygen atoms. However, H2O3 decomposes extremely readily in water to form (1)O2 and water, rather than O3 and H2O2. This article highlights key literature indicating that the oxidation of organic molecules such as the amino acids methionine, tryptophan, histidine, and cysteine by (1)O2 is involved in ozone formation. Based on this, an alternative hypothesis for ozone formation is developed involving a further reaction of singlet oxygen with various oxidized organic intermediates. H2O2 having water-derived oxygen atoms is subsequently formed during ozone decomposition in water by known reactions.
Keywords: Amino acid oxidation; Baeyer-Villiger oxidation; Hydrogen peroxide; Ozone; Singlet oxygen.
Figures
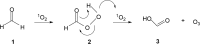



Similar articles
-
The competition between cathodic oxygen and ozone reduction and its role in dictating the reaction mechanisms of an electro-peroxone process.Water Res. 2017 Jul 1;118:26-38. doi: 10.1016/j.watres.2017.04.005. Epub 2017 Apr 4. Water Res. 2017. PMID: 28412550
-
Formation of hydrogen polyoxides as constituents of peroxy radical condensate upon low-temperature interaction of hydrogen atoms with liquid ozone.J Phys Chem A. 2014 Jan 9;118(1):62-9. doi: 10.1021/jp410938b. Epub 2013 Dec 23. J Phys Chem A. 2014. PMID: 24325583
-
Comparison of pharmaceutical abatement in various water matrices by conventional ozonation, peroxone (O3/H2O2), and an electro-peroxone process.Water Res. 2018 Mar 1;130:127-138. doi: 10.1016/j.watres.2017.11.054. Epub 2017 Nov 27. Water Res. 2018. PMID: 29216480
-
Interconnection of reactive oxygen species chemistry across the interfaces of atmospheric, environmental, and biological processes.Acc Chem Res. 2015 Mar 17;48(3):575-83. doi: 10.1021/ar500412p. Epub 2015 Feb 17. Acc Chem Res. 2015. PMID: 25688469 Review.
-
Implications of cholesterol autoxidation products in the pathogenesis of inflammatory diseases.Biochem Biophys Res Commun. 2014 Apr 11;446(3):702-8. doi: 10.1016/j.bbrc.2013.12.107. Epub 2014 Jan 9. Biochem Biophys Res Commun. 2014. PMID: 24412245 Review.
Cited by
-
The Contribution of Singlet Oxygen to Insulin Resistance.Oxid Med Cell Longev. 2017;2017:8765972. doi: 10.1155/2017/8765972. Epub 2017 Sep 7. Oxid Med Cell Longev. 2017. PMID: 29081894 Free PMC article. Review.
-
Endogenous Generation of Singlet Oxygen and Ozone in Human and Animal Tissues: Mechanisms, Biological Significance, and Influence of Dietary Components.Oxid Med Cell Longev. 2016;2016:2398573. doi: 10.1155/2016/2398573. Epub 2016 Mar 6. Oxid Med Cell Longev. 2016. PMID: 27042259 Free PMC article. Review.
-
Cellular Stresses and Stress Responses in the Pathogenesis of Insulin Resistance.Oxid Med Cell Longev. 2018 Jul 9;2018:4321714. doi: 10.1155/2018/4321714. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30116482 Free PMC article. Review.
References
-
- Balla J, Tyihak E. Direct measurement of emission of endogenous ozone from plants by GC-MS-SIM. Chromatographia Supp. 2010;71:S87–S91. doi: 10.1365/s10337-010-1594-x. - DOI
-
- Beltran FJ (2004) Ozone reaction kinetics for water and wastewater systems. CRC Press, pp 13–14
-
- Wentworth P, Jr, McDunn JE, Wentworth AD, Takeuchi C, Nieva J, Jones T, Bautista C, Ruedi JM, Gutierrez A, Janda KD, Babior BM, Eschenmoser A, Lerner RA. Evidence for antibody-catalyzed ozone formation in bacterial killing and inflammation. Science. 2002;298:2195–2199. doi: 10.1126/science.1077642. - DOI - PubMed
-
- Pereira PMR. Antibodies armed with photosensitizers: from chemical synthesis to photobiological applications. Org Biomol Chem. 2015 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

