Mavoglurant in adolescents with fragile X syndrome: analysis of Clinical Global Impression-Improvement source data from a double-blind therapeutic study followed by an open-label, long-term extension study
- PMID: 26855682
- PMCID: PMC4743124
- DOI: 10.1186/s11689-015-9134-5
Mavoglurant in adolescents with fragile X syndrome: analysis of Clinical Global Impression-Improvement source data from a double-blind therapeutic study followed by an open-label, long-term extension study
Abstract
Background: A phase II randomized, placebo-controlled, double-blind study and subsequent open-label extension study evaluated the efficacy, safety, and tolerability of mavoglurant (AFQ056), a selective metabotropic glutamate receptor subtype-5 antagonist, in treating behavioral symptoms in adolescent patients with fragile X syndrome (FXS). A novel method was applied to analyze changes in symptom domains in patients with FXS using the narratives associated with the clinician-rated Clinical Global Impression-Improvement (CGI-I) scale.
Methods: In the core study, patients were randomized to receive mavoglurant (25, 50, or 100 mg BID) or placebo over 12 weeks. In the extension, patients received 100 mg BID mavoglurant (or the highest tolerated dose) for up to 32 months. Global improvement, as a measure of treatment response, was assessed using the CGI-I scale. Investigators assigning CGI-I scores of 1 (very much improved), 2 (much improved), 6 (much worse), or 7 (very much worse) were provided a standard narrative template to collect further information about the changes observed in patients. Investigator feedback was coded and clustered into categories of improvement or worsening to identify potential areas of improvement with mavoglurant. Treatment effect in each category was characterized using the Cochran-Mantel-Haenszel test.
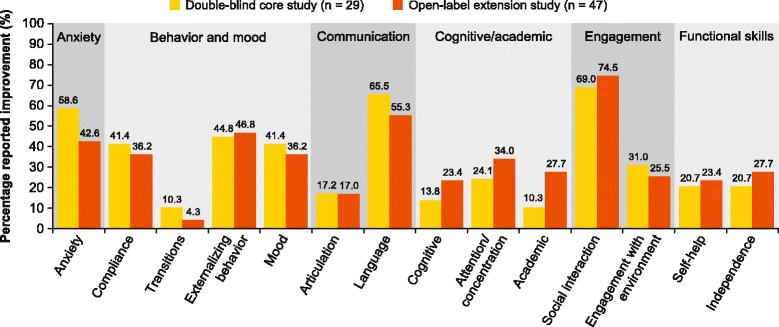
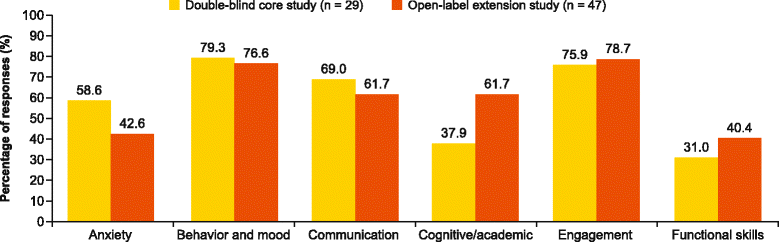
Results: A total of 134 and 103 patients had reached 2 weeks or more of core and extension study treatment, respectively, by the pre-assigned cutoff date for investigator feedback. In the core study, 34 CGI-I scores of 1 or 2 were reported in 28 patients; one patient scored 6. Analysis of the CGI-I narratives did not indicate greater treatment response in patients receiving mavoglurant compared with placebo in any specific improvement domain. There were 54 CGI-I scores of 1 or 2 in 47 patients in the extension study. The most frequently reported categories of improvement were behavior and mood (79.3 and 76.6 % in core and extension studies, respectively), engagement (75.9 and 78.7 %), and communication (69.0 and 61.7 %).
Conclusions: A method was established to capture and categorize FXS symptoms using CGI-I narratives. Although this method did not show benefit of drug over placebo, narratives from investigators were mostly based on parental report and thus do not represent a completely objective alternative assessment.
Trial registration: The studies described are registered at ClinicalTrials.gov with clinical trial identifier numbers NCT01357239 and NCT01433354.
Keywords: AFQ056; CGI-I; Clinical Global Impression-Improvement; Fragile X syndrome; Mavoglurant.
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical