The status of glucocorticoid-induced leucine zipper protein in the salivary glands in Sjögren's syndrome: predictive and prognostic potentials
- PMID: 26855686
- PMCID: PMC4743166
- DOI: 10.1186/s13167-016-0052-8
The status of glucocorticoid-induced leucine zipper protein in the salivary glands in Sjögren's syndrome: predictive and prognostic potentials
Abstract
Background: We recently showed that an imbalance between the pro-inflammatory cytokine, interleukin (IL)-17, and the developmental endothelial locus-1 (Del-1) likely contributes to inflammation and salivary gland abnormalities in Sjögren's syndrome (SS). The glucocorticoid-induced leucine zipper (GILZ) protein is a pivotal player in mediating the anti-inflammatory effects of glucocorticoids. However, its status and role in salivary gland inflammation and dysfunction in SS are not established. Thus, we tested the hypothesis that SS is associated with reduced GILZ expression, thereby contributing to Del-1/Il-17 imbalance and inflammation in salivary glands.
Methods: We utilized the nonobese diabetic (NOD) mice, a model of SS-like disease as well as lower-lip biopsy samples of subjects without or with a diagnosis of SS in association with immunostaining studies. These studies were complemented with in vitro and flow-cytometry studies whereby interleukin (IL)-23-treated salivary gland cells were co-cultured with GILZ-expressing cells or control cells; IL-23 is known to increase generation of IL-17.
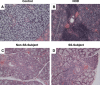
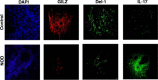
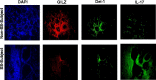
Results: Salivary glands of NOD mice displayed marked leukocyte infiltration and reduced GILZ expression in association with increased IL-17 but decreased Del-1 expression. A similar pattern was observed for lower-lip biopsy samples of SS than non-SS subjects. Further, IL-23-treated salivary gland cells displayed marked increase in IL-17 but reduced Del-1 positive cells which were reversed with co-culturing with GILZ-expressing cells but not control cells. Collectively, the results are suggestive of dysregulation of GILZ playing a role in inflammation and associated Del-1/Il-17 imbalance in SS.
Conclusions: Complementing our demonstration of Del-1/IL-17 imbalance in salivary glands in SS, the present study has established the relevance and significance of GILZ as a novel predictive and prognostic molecular fingerprint for SS. Thus, assessment of minor salivary gland GILZ expression, in conjunction with Del-1/IL-17 imbalance, could potentially offer a more sensitive approach to help with diagnosis of SS, at early stage of the disease, than that based on leukocyte infiltration. Future studies should establish whether treatment with GILZ ameliorates signs and symptoms of salivary malfunction of SS for which effective treatment options remain elusive.
Keywords: GILZ; Inflammation; Predictive preventive and personalized medicine; Salivary glands; Sjögren’s syndrome.
Figures


Similar articles
-
Reciprocal relation between GADD153 and Del-1 in regulation of salivary gland inflammation in Sjögren syndrome.Exp Mol Pathol. 2013 Dec;95(3):288-97. doi: 10.1016/j.yexmp.2013.09.002. Epub 2013 Sep 20. Exp Mol Pathol. 2013. PMID: 24060278
-
Interleukin-17 Impairs Salivary Tight Junction Integrity in Sjögren's Syndrome.J Dent Res. 2016 Jul;95(7):784-92. doi: 10.1177/0022034516634647. Epub 2016 Mar 1. J Dent Res. 2016. PMID: 26933138
-
Mesenchymal stem cell transplantation ameliorates Sjögren's syndrome via suppressing IL-12 production by dendritic cells.Stem Cell Res Ther. 2018 Nov 8;9(1):308. doi: 10.1186/s13287-018-1023-x. Stem Cell Res Ther. 2018. PMID: 30409219 Free PMC article.
-
The Potential Role for Early Biomarker Testing as Part of a Modern, Multidisciplinary Approach to Sjögren's Syndrome Diagnosis.Adv Ther. 2017 Apr;34(4):799-812. doi: 10.1007/s12325-017-0501-3. Epub 2017 Mar 10. Adv Ther. 2017. PMID: 28283891 Review.
-
Recent Advances of Salivary Gland Biopsy in Sjögren's Syndrome.Front Med (Lausanne). 2022 Jan 10;8:792593. doi: 10.3389/fmed.2021.792593. eCollection 2021. Front Med (Lausanne). 2022. PMID: 35083248 Free PMC article. Review.
Cited by
-
Efficacy of nanoceria for periodontal tissues alteration in glutamate-induced obese rats-multidisciplinary considerations for personalized dentistry and prevention.EPMA J. 2017 Mar 14;8(1):43-49. doi: 10.1007/s13167-017-0085-7. eCollection 2017 Mar. EPMA J. 2017. PMID: 28620442 Free PMC article.
-
Glucocorticoid-Induced Leucine Zipper (GILZ) in Cardiovascular Health and Disease.Cells. 2021 Aug 21;10(8):2155. doi: 10.3390/cells10082155. Cells. 2021. PMID: 34440924 Free PMC article. Review.
-
Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016.EPMA J. 2016 Oct 25;7(1):23. doi: 10.1186/s13167-016-0072-4. eCollection 2016. EPMA J. 2016. PMID: 27800037 Free PMC article. Review. No abstract available.
-
The potential relationship between Flammer and Sjögren syndromes: the chime of dysfunction.EPMA J. 2017 Aug 14;8(4):333-338. doi: 10.1007/s13167-017-0107-5. eCollection 2017 Dec. EPMA J. 2017. PMID: 29209436 Free PMC article. Review.
-
Growth Hormone(s), Testosterone, Insulin-Like Growth Factors, and Cortisol: Roles and Integration for Cellular Development and Growth With Exercise.Front Endocrinol (Lausanne). 2020 Feb 25;11:33. doi: 10.3389/fendo.2020.00033. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32158429 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

