A novel fibre-ensemble level constitutive model for exogenous cross-linked collagenous tissues
- PMID: 26855761
- PMCID: PMC4686250
- DOI: 10.1098/rsfs.2015.0090
A novel fibre-ensemble level constitutive model for exogenous cross-linked collagenous tissues
Abstract
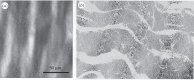
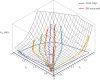
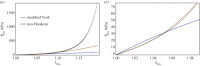
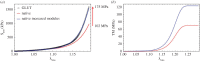
Exogenous cross-linking of soft collagenous tissues is a common method for biomaterial development and medical therapies. To enable improved applications through computational methods, physically realistic constitutive models are required. Yet, despite decades of research, development and clinical use, no such model exists. In this study, we develop the first rigorous full structural model (i.e. explicitly incorporating various features of the collagen fibre architecture) for exogenously cross-linked soft tissues. This was made possible, in-part, with the use of native to cross-linked matched experimental datasets and an extension to the collagenous structural constitutive model so that the uncross-linked collagen fibre responses could be mapped to the cross-linked configuration. This allowed us to separate the effects of cross-linking from kinematic changes induced in the cross-linking process, which in turn allowed the non-fibrous tissue matrix component and the interaction effects to be identified. It was determined that the matrix could be modelled as an isotropic material using a modified Yeoh model. The most novel findings of this study were that: (i) the effective collagen fibre modulus was unaffected by cross-linking and (ii) fibre-ensemble interactions played a large role in stress development, often dominating the total tissue response (depending on the stress component and loading path considered). An important utility of the present model is its ability to separate the effects of exogenous cross-linking on the fibres from changes due to the matrix. Applications of this approach include the utilization in the design of novel chemical treatments to produce specific mechanical responses and the study of fatigue damage in bioprosthetic heart valve biomaterials.
Keywords: collagenous tissues; constitutive model; cross-linking.
Figures







References
-
- Schoen FJ, Levy RJ. 1999. Founder's Award, 25th Annual Meeting of the Society for Biomaterials, perspectives. Providence, RI, April 28-May 2, 1999. Tissue heart valves: current challenges and future research perspectives. J. Biomed. Mater. Res. 47, 439–465. (10.1002/(SICI)1097-4636(19991215)47:4<439::AID-JBM1>3.0.CO;2-O) - DOI - PubMed
-
- Vesely I, Barber JE, Ratliff NB. 2001. Tissue damage and calcification may be independent mechanisms of bioprosthetic heart valve failure. J. Heart Valve Dis. 10, 471–477. - PubMed
-
- Sacks MS, Schoen FJ (eds). 1999. Calcification-independent collagen damage in explanted clinical bioprosthetic heart valves. Providence, RI: Society for Biomaterials.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

