Alternating bipolar field stimulation identifies muscle fibers with defective excitability but maintained local Ca(2+) signals and contraction
- PMID: 26855765
- PMCID: PMC4743112
- DOI: 10.1186/s13395-016-0076-8
Alternating bipolar field stimulation identifies muscle fibers with defective excitability but maintained local Ca(2+) signals and contraction
Abstract
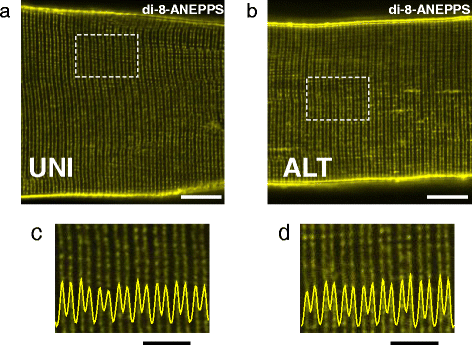
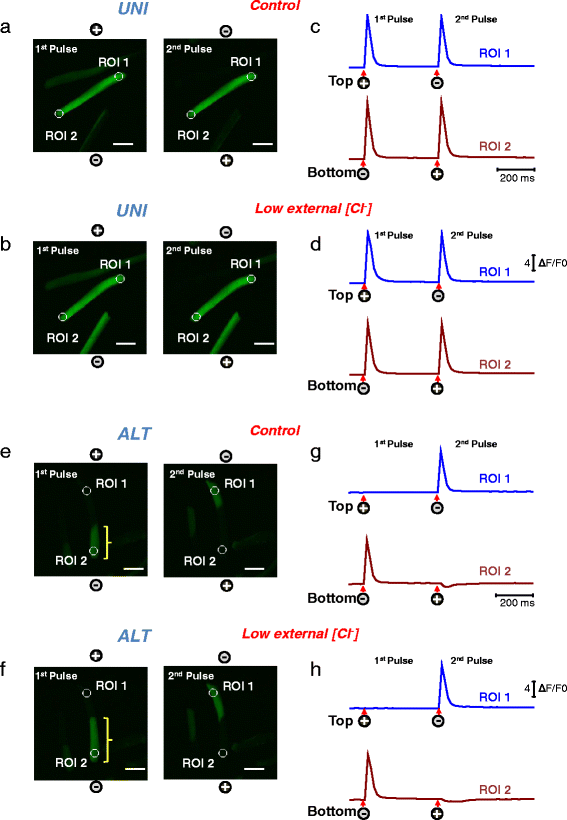
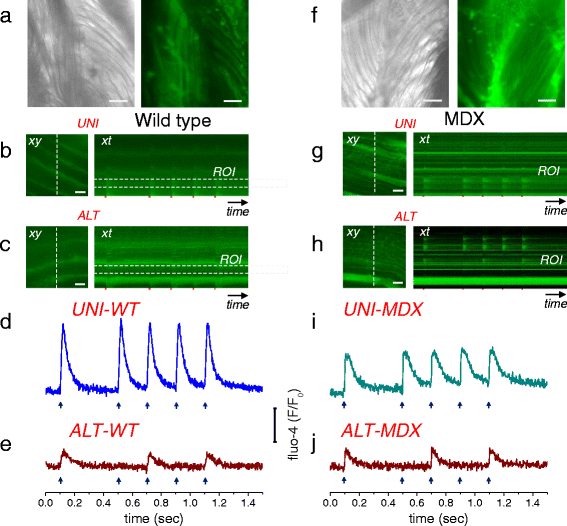
Background: Most cultured enzymatically dissociated adult myofibers exhibit spatially uniform (UNI) contractile responses and Ca(2+) transients over the entire myofiber in response to electric field stimuli of either polarity applied via bipolar electrodes. However, some myofibers only exhibit contraction and Ca(2+) transients at alternating (ALT) ends in response to alternating polarity field stimulation. Here, we present for the first time the methodology for identification of ALT myofibers in primary cultures and isolated muscles, as well as a study of their electrophysiological properties.
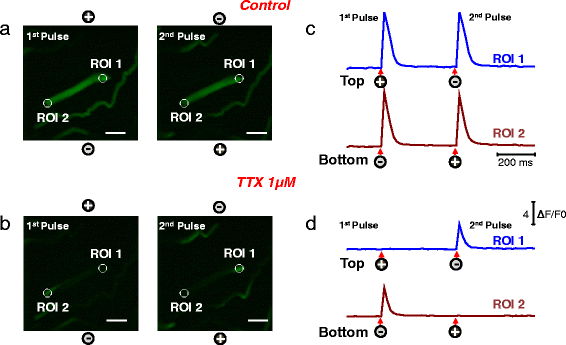
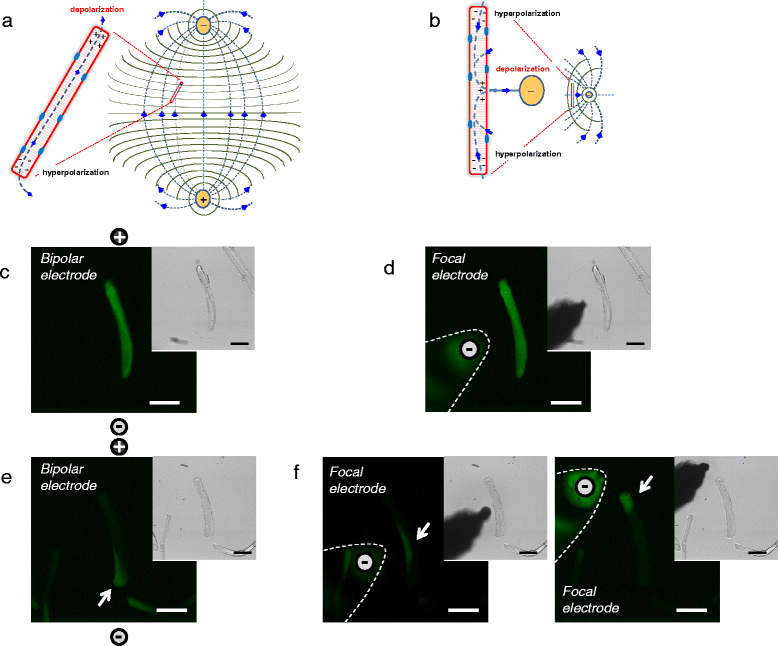
Results: We used high-speed confocal microscopic Ca(2+) imaging, electric field stimulation, microelectrode recordings, immunostaining, and confocal microscopy to characterize the properties of action potential-induced Ca(2+) transients, contractility, resting membrane potential, and staining of T-tubule voltage-gated Na(+) channel distribution applied to cultured adult myofibers. Here, we show for the first time, with high temporal and spatial resolution, that normal control myofibers with UNI responses can be converted to ALT response myofibers by TTX addition or by removal of Na(+) from the bathing medium, with reappearance of the UNI response on return of Na(+). Our results suggest disrupted excitability as the cause of ALT behavior and indicate that the ALT response is due to local depolarization-induced Ca(2+) release, whereas the UNI response is triggered by action potential propagation over the entire myofiber. Consistent with this interpretation, local depolarizing monopolar stimuli give uniform (propagated) responses in UNI myofibers, but only local responses at the electrode in ALT myofibers. The ALT responses in electrically inexcitable myofibers are consistent with expectations of current spread between bipolar stimulating electrodes, entering (hyperpolarizing) one end of a myofiber and leaving (depolarizing) the other end of the myofiber. ALT responses were also detected in some myofibers within intact isolated whole muscles from wild-type and MDX mice, demonstrating that ALT responses can be present before enzymatic dissociation.
Conclusions: We suggest that checking for ALT myofiber responsiveness by looking at the end of a myofiber during alternating polarity stimuli provides a test for compromised excitability of myofibers, and could be used to identify inexcitable, damaged or diseased myofibers by ALT behavior in healthy and diseased muscle.
Keywords: Abnormal excitability; Cultured myofibers; Enzymatic dissociation; Excitation-contraction coupling; Skeletal muscle.
Figures



Similar articles
-
Malformed mdx myofibers have normal cytoskeletal architecture yet altered EC coupling and stress-induced Ca2+ signaling.Am J Physiol Cell Physiol. 2009 Sep;297(3):C571-80. doi: 10.1152/ajpcell.00087.2009. Epub 2009 Jul 15. Am J Physiol Cell Physiol. 2009. PMID: 19605736 Free PMC article.
-
Attenuated Ca(2+) release in a mouse model of limb girdle muscular dystrophy 2A.Skelet Muscle. 2016 Feb 24;6:11. doi: 10.1186/s13395-016-0081-y. eCollection 2016. Skelet Muscle. 2016. PMID: 26913171 Free PMC article.
-
Disruption of action potential and calcium signaling properties in malformed myofibers from dystrophin-deficient mice.Physiol Rep. 2015 Apr;3(4):e12366. doi: 10.14814/phy2.12366. Physiol Rep. 2015. PMID: 25907787 Free PMC article.
-
Ion channels and ion transporters of the transverse tubular system of skeletal muscle.J Muscle Res Cell Motil. 2006;27(5-7):275-90. doi: 10.1007/s10974-006-9088-z. Epub 2006 Aug 24. J Muscle Res Cell Motil. 2006. PMID: 16933023 Review.
-
Signaling complexes of voltage-gated sodium and calcium channels.Neurosci Lett. 2010 Dec 10;486(2):107-16. doi: 10.1016/j.neulet.2010.08.085. Epub 2010 Sep 17. Neurosci Lett. 2010. PMID: 20816922 Free PMC article. Review.
Cited by
-
Cardioprotective Effect of Monoammonium Glycyrrhizinate Injection Against Myocardial Ischemic Injury in vivo and in vitro: Involvement of Inhibiting Oxidative Stress and Regulating Ca2+ Homeostasis by L-Type Calcium Channels.Drug Des Devel Ther. 2020 Jan 23;14:331-346. doi: 10.2147/DDDT.S232130. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32158189 Free PMC article.
-
Optical Recording of Action Potential Initiation and Propagation in Mouse Skeletal Muscle Fibers.Biophys J. 2018 Dec 4;115(11):2127-2140. doi: 10.1016/j.bpj.2018.10.026. Epub 2018 Nov 3. Biophys J. 2018. PMID: 30448039 Free PMC article.
-
The role of action potential changes in depolarization-induced failure of excitation contraction coupling in mouse skeletal muscle.Elife. 2022 Jan 5;11:e71588. doi: 10.7554/eLife.71588. Elife. 2022. PMID: 34985413 Free PMC article.
-
Voltage sensor movements of CaV1.1 during an action potential in skeletal muscle fibers.Proc Natl Acad Sci U S A. 2021 Oct 5;118(40):e2026116118. doi: 10.1073/pnas.2026116118. Proc Natl Acad Sci U S A. 2021. PMID: 34583989 Free PMC article.
-
Acute Elevated Glucose Promotes Abnormal Action Potential-Induced Ca2+ Transients in Cultured Skeletal Muscle Fibers.J Diabetes Res. 2017;2017:1509048. doi: 10.1155/2017/1509048. Epub 2017 Aug 1. J Diabetes Res. 2017. PMID: 28835899 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

