Sperm performance in conspecific and heterospecific female fluid
- PMID: 26855769
- PMCID: PMC4733106
- DOI: 10.1002/ece3.1977
Sperm performance in conspecific and heterospecific female fluid
Abstract
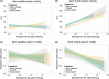
Divergent sexual selection within allopatric populations may result in divergent sexual phenotypes, which can act as reproductive barriers between populations upon secondary contact. This hypothesis has been most tested on traits involved in precopulatory sexual selection, with less work focusing on traits that act after copulation and before fertilization (i.e., postcopulatory prezygotic traits), particularly in internally fertilizing vertebrates. However, postcopulatory sexual selection within species can also drive trait divergence, resulting in reduced performance of heterospecific sperm within the female reproductive tract. Such incompatibilities, arising as a by-product of divergent postcopulatory sexual selection in allopatry, can represent reproductive barriers, analogous to species-assortative mating preferences. Here, we tested for postcopulatory prezygotic reproductive barriers between three pairs of taxa with diverged sperm phenotypes and moderate-to-high opportunity for postcopulatory sexual selection (barn swallows Hirundo rustica versus sand martins Riparia riparia, two subspecies of bluethroats, Luscinia svecica svecica versus L. s. namnetum, and great tits Parus major versus blue tits Cyanistes caeruleus). We tested sperm swimming performance in fluid from the outer reproductive tract of females, because the greatest reduction in sperm number in birds occurs as sperm swim across the vagina. Contrary to our expectations, sperm swam equally well in fluid from conspecific and heterospecific females, suggesting that postcopulatory prezygotic barriers do not act between these taxon pairs, at this stage between copulation and fertilization. We therefore suggest that divergence in sperm phenotypes in allopatry is insufficient to cause widespread postcopulatory prezygotic barriers in the form of impaired sperm swimming performance in passerine birds.
Keywords: Cryptic female choice; postcopulatory prezygotic barriers; sexual selection; speciation; sperm competition; sperm motility; sperm velocity.
Figures


References
-
- Amann, R. P. , and Waberski D.. 2014. Computer‐assisted sperm analysis (CASA): capabilities and potential developments. Theriogenology 81:5–17.e1–3. - PubMed
-
- Augustin, J. , Blomqvist D., Szép T., Szabó Z. D., and Wagner R. H.. 2006. No evidence of genetic benefits from extra‐pair fertilisations in female sand martins (Riparia riparia). J. Ornithol. 148:189–198.
-
- Bakst, M. R. , Wishart G., and Brillard J.‐P.. 1994. Oviductal sperm selection, transport, and storage in poultry. Poult. Sci. Rev. 5:117–143.
-
- Bates, D. , Maechler M., Bolker B., and Walker S.. 2015. Fitting linear mixed‐effects models using lme4. J Stat Software 67:1–48.
-
- Beirão, J. , Purchase C., Wringe B., and Fleming I.. 2014. Wild Atlantic cod sperm motility is negatively affected by ovarian fluid of farmed females. Aquac. Environ. Interact. 5:61–70.
LinkOut - more resources
Full Text Sources
Other Literature Sources

