Basal body structure and composition in the apicomplexans Toxoplasma and Plasmodium
- PMID: 26855772
- PMCID: PMC4743101
- DOI: 10.1186/s13630-016-0025-5
Basal body structure and composition in the apicomplexans Toxoplasma and Plasmodium
Abstract
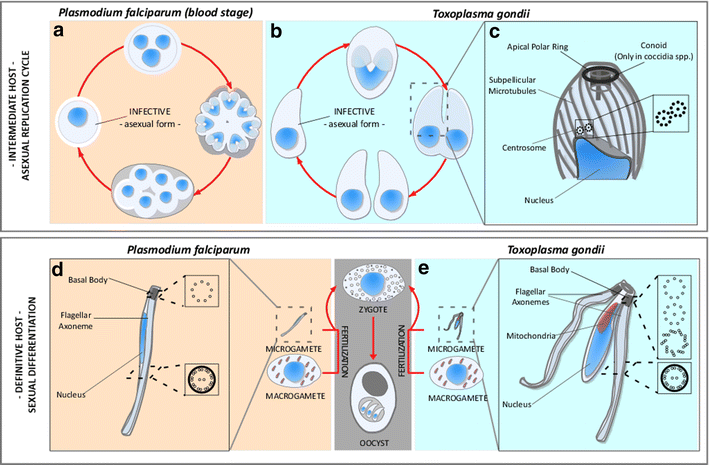
The phylum Apicomplexa encompasses numerous important human and animal disease-causing parasites, including the Plasmodium species, and Toxoplasma gondii, causative agents of malaria and toxoplasmosis, respectively. Apicomplexans proliferate by asexual replication and can also undergo sexual recombination. Most life cycle stages of the parasite lack flagella; these structures only appear on male gametes. Although male gametes (microgametes) assemble a typical 9+2 axoneme, the structure of the templating basal body is poorly defined. Moreover, the relationship between asexual stage centrioles and microgamete basal bodies remains unclear. While asexual stages of Plasmodium lack defined centriole structures, the asexual stages of Toxoplasma and closely related coccidian apicomplexans contain centrioles that consist of nine singlet microtubules and a central tubule. There are relatively few ultra-structural images of Toxoplasma microgametes, which only develop in cat intestinal epithelium. Only a subset of these include sections through the basal body: to date, none have unambiguously captured organization of the basal body structure. Moreover, it is unclear whether this basal body is derived from pre-existing asexual stage centrioles or is synthesized de novo. Basal bodies in Plasmodium microgametes are thought to be synthesized de novo, and their assembly remains ill-defined. Apicomplexan genomes harbor genes encoding δ- and ε-tubulin homologs, potentially enabling these parasites to assemble a typical triplet basal body structure. Moreover, the UNIMOD components (SAS6, SAS4/CPAP, and BLD10/CEP135) are conserved in these organisms. However, other widely conserved basal body and flagellar biogenesis elements are missing from apicomplexan genomes. These differences may indicate variations in flagellar biogenesis pathways and in basal body arrangement within the phylum. As apicomplexan basal bodies are distinct from their metazoan counterparts, it may be possible to selectively target parasite structures in order to inhibit microgamete motility which drives generation of genetic diversity in Toxoplasma and transmission for Plasmodium.
Keywords: Centriole; Coccidia; Flagellum; Malaria; Microgamete; Microtubule organizing center.
Figures
Similar articles
-
The Tubulin Superfamily in Apicomplexan Parasites.Microorganisms. 2023 Mar 9;11(3):706. doi: 10.3390/microorganisms11030706. Microorganisms. 2023. PMID: 36985278 Free PMC article.
-
Structural and Functional Insights into the Microtubule Organizing Centers of Toxoplasma gondii and Plasmodium spp.Microorganisms. 2021 Dec 3;9(12):2503. doi: 10.3390/microorganisms9122503. Microorganisms. 2021. PMID: 34946106 Free PMC article. Review.
-
Expansion microscopy of Plasmodium gametocytes reveals the molecular architecture of a bipartite microtubule organisation centre coordinating mitosis with axoneme assembly.PLoS Pathog. 2022 Jan 25;18(1):e1010223. doi: 10.1371/journal.ppat.1010223. eCollection 2022 Jan. PLoS Pathog. 2022. PMID: 35077503 Free PMC article.
-
Plasmodium SAS4: basal body component of male cell which is dispensable for parasite transmission.Life Sci Alliance. 2022 May 12;5(9):e202101329. doi: 10.26508/lsa.202101329. Print 2022 Sep. Life Sci Alliance. 2022. PMID: 35550346 Free PMC article.
-
The Riveting Cellular Structures of Apicomplexan Parasites.Trends Parasitol. 2020 Dec;36(12):979-991. doi: 10.1016/j.pt.2020.09.001. Epub 2020 Sep 30. Trends Parasitol. 2020. PMID: 33011071 Review.
Cited by
-
Unravelling the sexual developmental biology of Cystoisospora suis, a model for comparative coccidian parasite studies.Front Cell Infect Microbiol. 2023 Oct 25;13:1271731. doi: 10.3389/fcimb.2023.1271731. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37953800 Free PMC article.
-
Melittin as a promising anti-protozoan peptide: current knowledge and future prospects.AMB Express. 2021 May 13;11(1):69. doi: 10.1186/s13568-021-01229-1. AMB Express. 2021. PMID: 33983454 Free PMC article. Review.
-
Real-time dynamics of Plasmodium NDC80 reveals unusual modes of chromosome segregation during parasite proliferation.J Cell Sci. 2020 Jun 30;134(5):jcs245753. doi: 10.1242/jcs.245753. J Cell Sci. 2020. PMID: 32501284 Free PMC article.
-
An axonemal intron splicing program sustains Plasmodium male development.Nat Commun. 2024 Jun 1;15(1):4697. doi: 10.1038/s41467-024-49002-9. Nat Commun. 2024. PMID: 38824128 Free PMC article.
-
The Structural and Molecular Underpinnings of Gametogenesis in Toxoplasma gondii.Front Cell Infect Microbiol. 2020 Dec 7;10:608291. doi: 10.3389/fcimb.2020.608291. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33365279 Free PMC article. Review.
References
-
- Adl SM, Simpson AGB, Farmer MA, Andersen RA, Anderson OR, Barta JR, Bowser SS, Brugerolle G, Fensome RA, Fredericq S, et al. The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J Eukaryot Microbiol. 2005;52:399–451. doi: 10.1111/j.1550-7408.2005.00053.x. - DOI - PubMed
-
- Burke-Gaffney HJ. Malaria. Trop Dis Bull. 1964;61:329–356. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

