Light-Sensitive Ruthenium Complex-Loaded Cross-linked Polymeric Nanoassemblies for the Treatment of Cancer
- PMID: 26855780
- PMCID: PMC4739781
- DOI: 10.1039/C5TB01613D
Light-Sensitive Ruthenium Complex-Loaded Cross-linked Polymeric Nanoassemblies for the Treatment of Cancer
Abstract
This work focuses on improving the efficacy of photoactivatable Ru complexes for photodynamic therapy by employing cross-linked nanoassemblies (CNAs) as a delivery approach. The effects of complex photoactivation, hydrophobicity, and solution ionic strength and pH on complex loading and release from CNAs were analyzed. The cell cytotoxicity of CNA formulations was similar to free Ru complexes despite reduced or eliminated DNA interactions. The release rate and the amount of each Ru complex released (%) varied inversely with complex hydrophobicity, while the effect of solution ionic strength was dependent on complex hydrophobicity. Premature release of two photoactivatable prodrugs prior to irradiation was believed to account for higher activity in cells studies compared to DNA interaction studies; however, for photostable 1O2 generator-loaded CNAs this cannot explain the high cytotoxicity and lack of DNA interactions because release was incomplete after 48 hrs. The cause remains unclear, but among other possibilities, accelerated release in a cell culture environment may be responsible.
Figures


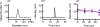
 ) and 2-loaded PEG-ASP CNAs (
) and 2-loaded PEG-ASP CNAs (
 )).
)).

 ) and light irradiated PEG-ASP CNAs (
) and light irradiated PEG-ASP CNAs (
 )) compared to free Ru complexes released from 10K MWCO dialysis cassettes (
)) compared to free Ru complexes released from 10K MWCO dialysis cassettes (
 ). Because 3 was a photostable Ru complex, release was only evaluated when protected from light. The MLCT peaks of the entrapped Ru complexes were monitored in order to determine the amount of each Ru complex remaining inside the PEG-ASP CNAs, and this was used to calculate the amount of Ru complexes released as a function of time (N = 3).
). Because 3 was a photostable Ru complex, release was only evaluated when protected from light. The MLCT peaks of the entrapped Ru complexes were monitored in order to determine the amount of each Ru complex remaining inside the PEG-ASP CNAs, and this was used to calculate the amount of Ru complexes released as a function of time (N = 3).
 ), dark phosphate buffer (
), dark phosphate buffer (
 ), dark PBS (
), dark PBS (
 ), light phosphate buffer (
), light phosphate buffer (
 ), and light PBS (
), and light PBS (
 )); d) shows a magnification of the rapid release of 3 from PEG-ASP CNAs when placed in a solution with low ionic strength (0 mM NaCl) (N = 3). Because 3 was a photostable Ru complex, release was only evaluated when protected from light.
)); d) shows a magnification of the rapid release of 3 from PEG-ASP CNAs when placed in a solution with low ionic strength (0 mM NaCl) (N = 3). Because 3 was a photostable Ru complex, release was only evaluated when protected from light.
 ), dark pH 7.4 (
), dark pH 7.4 (
 ), light pH 6.0 (
), light pH 6.0 (
 ), and light pH 7.4 (
), and light pH 7.4 (
 )) compared to the release of free complexes from 10K MWCO dialysis cassettes (
)) compared to the release of free complexes from 10K MWCO dialysis cassettes (
 ) (N = 3). Because 3 was a photostable Ru complex, release was only evaluated when protected from light (N = 3).
) (N = 3). Because 3 was a photostable Ru complex, release was only evaluated when protected from light (N = 3).

References
-
- Howerton BS, Heidary DK, Glazer EC. Strained ruthenium complexes are potent light-activated anticancer agents. J Am Chem Soc. 2012;134:8324–8327. - PubMed
- Dickerson M, Sun Y, Howerton B, Glazer EC. Modifying charge and hydrophilicity of simple Ru(II) polypyridyl complexes radically alters biological activities: old complexes, surprising new tricks. Inorg Chem. 2014;53(19):10370–7. - PMC - PubMed
-
- Zhang CX, Lippard SJ. New metal complexes as potential therapeutics. Curr Opin Chem Biol. 2003;7:481–489. - PubMed
- Fricker SP. Metal based drugs: from serendipity to design. Dalton Trans. 2007;43:4903–4917. - PubMed
- Bruijnincx PCA, Sadler PJ. New trends for metal complexes with anticancer activity. Curr Opin Chem Biol. 2008;12:197–206. - PMC - PubMed
- Chen T, Liu Y, Zheng WJ, Liu J, Wong YS. Ruthenium polypyridyl complexes that induce mitochondria-mediated apoptosis in cancer cells. Inorg Chem. 2010;49:6366–6368. - PubMed
- Groessl M, Zava O, Dyson PJ. Cellular uptake and subcellular distribution of ruthenium-based metallodrugs under clinical investigation versus cisplatin. Metallomics. 2011;3:591–599. - PubMed
-
- Goldbach RE, Rodriguez-Garcia I, van Lenthe JH, Siegler MA, Bonnet S. N-acetylmethionine and biotin as photocleavable protective groups for ruthenium polypyridyl complexes. Chem Eur J. 2011;17:9924–9929. - PubMed
- Wachter E, Heidary DK, Howerton BS, Parkin S, Glazer EC. Light-activated ruthenium complexes photobind DNA and are cytotoxic in the phtodynamic therapy window. Chem Commun. 2012;48:9649–9651. - PubMed
- Frasconi M, Liu Z, Lei J, Wu Y, Strekalova E, Malin D, Ambrogio MW, Chen X, Botros YY, Cryns VL, Sauvage JP, Stoddart JF. Photoexpulsion of surface-grafted ruthenium complexes and subsequent release of cytotoxic cargos to cancer cells from mesoporous silica nanoparticles. J Am Chem Soc. 2013;135:11603–11613. - PMC - PubMed
- Hufziger KT, Thowfeik FS, Charboneau DJ, Nieto I, Dougherty WG, Kassel WS, Dudley TJ, Merino EJ, Papish ET, Paul JJ. Ruthenium dihydroxybipyridine complexes are tumor activated prodrugs due to low pH and blue light induced ligand release. J Inorg Biochem. 2014;130:103–111. - PMC - PubMed
-
- Novakova O, Kasparkova J, Vrana O, van Vliet PM, Reedijk J, Brabec V. Correlation between cytotoxicity and DNA binding of polypyridyl ruthenium complexes. Biochemistry. 1995;34:12369–12378. - PubMed
- Kladjner M, Hebraud P, Sirlin C, Gaiddon C, Harlepp S. DNA binding to an anticancer organo-ruthenium complex. J Phys Chem. 2010;114:14041–14047. - PubMed
- Heidary DK, Glazer EC. A light-activated metal complex targets both DNA and RNA in a fluorescent in vitro transcription and translation assay. ChemBioChem. 2014;15:507–511. - PubMed
-
- Grover N, Welch TW, Fairley TA, Cory M, Thorp HH. Covalent Binding of Aquaruthenium complexes to DNA. Inorg Chem. 1994;33:3544–3548.
- Allardyce CS, Dyson PJ. Ruthenium in medicine: Current clinical uses and future prospects. Platinum Metals Rev. 2001;45:62–69.
- Lentzen O, Moucheron C, Kirsch-De Mesmaeker A. Metallotherapeutic drugs and metal-based diagnostic agents. John Wiley & Sons, Ltd; 2005. 44Ru Perspectives of ruthenium complexes in cancer therapy; pp. 359–378.
- Boerner LJK, Zaleski JM. Metal complex-DNA interactions: from transcription inhibition to photoactivated cleavage. Curr Opin Chem Biol. 2005;9:135–144. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

