Amphioxus mouth after dorso-ventral inversion
- PMID: 26855789
- PMCID: PMC4744632
- DOI: 10.1186/s40851-016-0038-3
Amphioxus mouth after dorso-ventral inversion
Abstract
Introduction: Deuterostomes (animals with 'secondary mouths') are generally accepted to develop the mouth independently of the blastopore. However, it remains largely unknown whether mouths are homologous among all deuterostome groups. Unlike other bilaterians, in amphioxus the mouth initially opens on the left lateral side. This peculiar morphology has not been fully explained in the evolutionary developmental context. We studied the developmental process of the amphioxus mouth to understand whether amphioxus acquired a new mouth, and if so, how it is related to or differs from mouths in other deuterostomes.
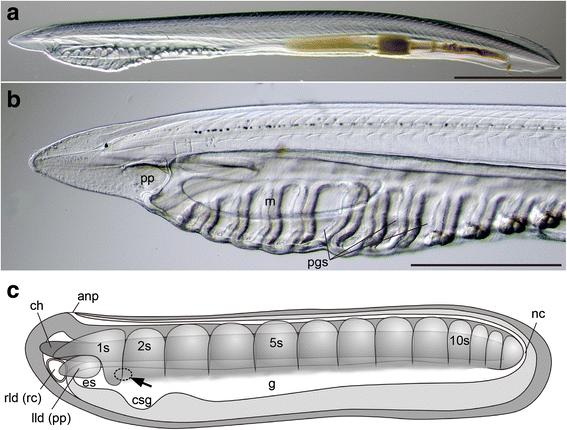
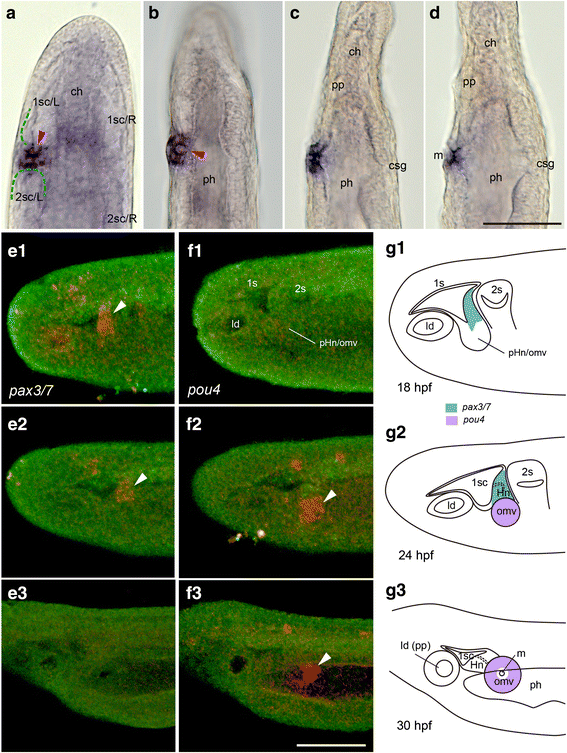
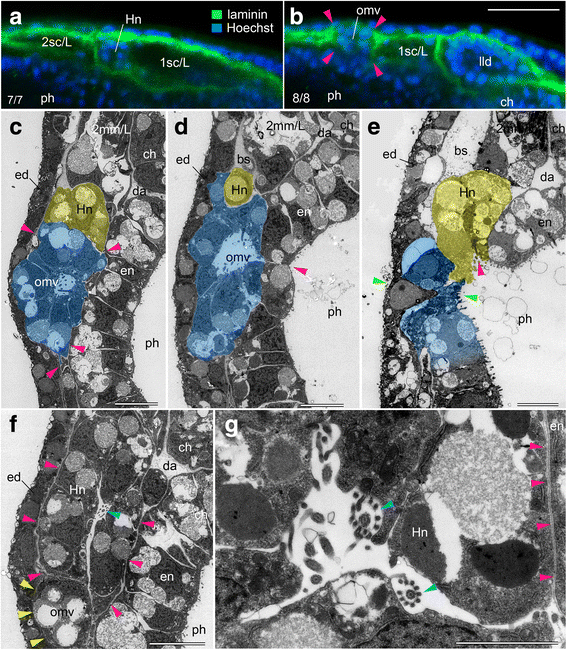
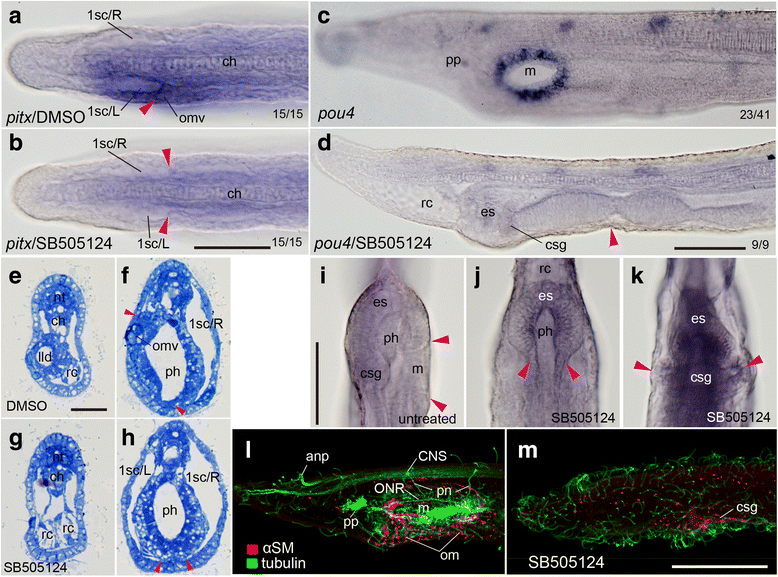
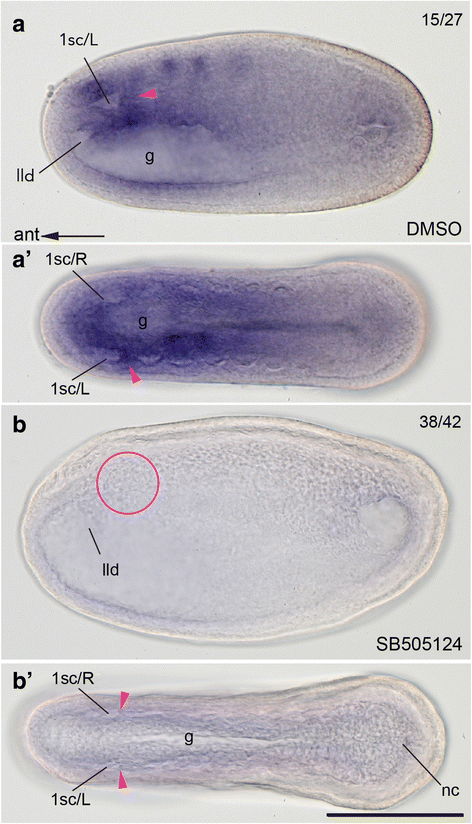
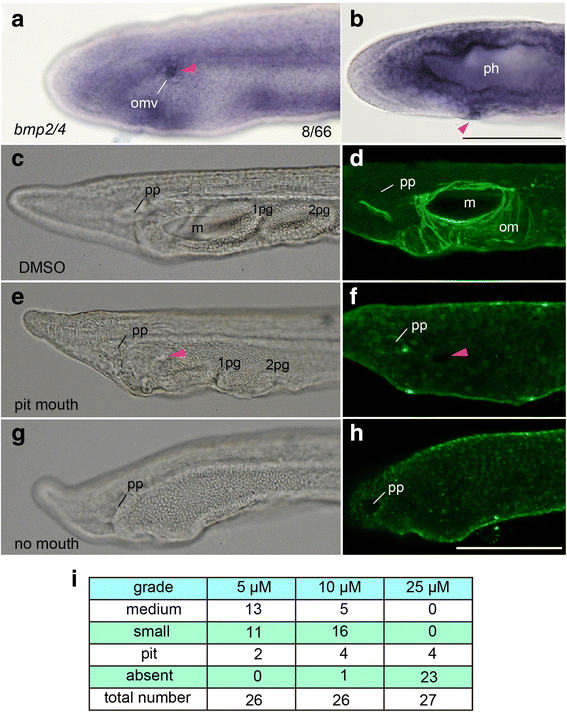
Results: The left first somite in amphioxus produces a coelomic vesicle between the epidermis and pharynx that plays a crucial role in the mouth opening. The vesicle develops in association with the amphioxus-specific Hatschek nephridium, and first opens into the pharynx and then into the exterior as a mouth. This asymmetrical development of the anterior-most somites depends on the Nodal-Pitx signaling unit, and the perturbation of laterality-determining Nodal signaling led to the disappearance of the vesicle, producing a symmetric pair of anterior-most somites that resulted in larvae lacking orobranchial structures. The vesicle expressed bmp2/4, as seen in ambulacrarian coelomic pore-canals, and the mouth did not open when Bmp2/4 signaling was blocked.
Conclusions: We conclude that the amphioxus mouth, which uniquely involves a mesodermal coelomic vesicle, shares its evolutionary origins with the ambulacrarian coelomic pore-canal. Our observations suggest that there are at least three types of mouths in deuterostomes, and that the new acquisition of chordate mouths was likely related to the dorso-ventral inversion that occurred in the last common ancestor of chordates.
Keywords: Coelom; Gill (branchial) slits; Homology of mouth; Hydropore; Lancelet; Nodal-signaling.
Figures



Comment in
-
Zoology: A New Mouth for Amphioxus.Curr Biol. 2016 May 9;26(9):R367-8. doi: 10.1016/j.cub.2016.03.016. Curr Biol. 2016. PMID: 27166696
Similar articles
-
The Nodal signaling pathway controls left-right asymmetric development in amphioxus.Evodevo. 2015 Feb 17;6:5. doi: 10.1186/2041-9139-6-5. eCollection 2015. Evodevo. 2015. PMID: 25954501 Free PMC article.
-
Tracing the Evolutionary Origin of Chordate Somites in the Hemichordate Ptychodera flava.Integr Comp Biol. 2024 Nov 21;64(5):1226-1242. doi: 10.1093/icb/icae020. Integr Comp Biol. 2024. PMID: 38637301
-
Acquisition of the dorsal structures in chordate amphioxus.Open Biol. 2016 Jun;6(6):160062. doi: 10.1098/rsob.160062. Open Biol. 2016. PMID: 27307516 Free PMC article.
-
Early development of amphioxus links evolutionary events with vertebrates.Int J Dev Biol. 2017;61(10-11-12):591-600. doi: 10.1387/ijdb.170118ky. Int J Dev Biol. 2017. PMID: 29319108 Review.
-
The lancelet and ammocoete mouths.Zoolog Sci. 2008 Oct;25(10):1012-9. doi: 10.2108/zsj.25.1012. Zoolog Sci. 2008. PMID: 19267637 Review.
Cited by
-
Evolution and loss of ß-catenin and TCF-dependent axis specification in insects.Curr Opin Insect Sci. 2022 Apr;50:100877. doi: 10.1016/j.cois.2022.100877. Epub 2022 Jan 31. Curr Opin Insect Sci. 2022. PMID: 35104659 Free PMC article. Review.
-
Optical Clearing and Light Sheet Microscopy Imaging of Amphioxus.Front Cell Dev Biol. 2021 Jul 26;9:702986. doi: 10.3389/fcell.2021.702986. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34381783 Free PMC article.
-
Cerberus-Nodal-Lefty-Pitx signaling cascade controls left-right asymmetry in amphioxus.Proc Natl Acad Sci U S A. 2017 Apr 4;114(14):3684-3689. doi: 10.1073/pnas.1620519114. Epub 2017 Mar 20. Proc Natl Acad Sci U S A. 2017. PMID: 28320954 Free PMC article.
-
Evolutionary history of the extant amphioxus lineage with shallow-branching diversification.Sci Rep. 2017 Apr 25;7(1):1157. doi: 10.1038/s41598-017-00786-5. Sci Rep. 2017. PMID: 28442709 Free PMC article.
-
Formation of the initial kidney and mouth opening in larval amphioxus studied with serial blockface scanning electron microscopy (SBSEM).Evodevo. 2018 Jun 21;9:16. doi: 10.1186/s13227-018-0104-3. eCollection 2018. Evodevo. 2018. PMID: 29977493 Free PMC article.
References
-
- Hejnol A, Martindale MQ. The mouth, the anus, and the blastopore --- open questions about questionable openings. In: Telford MJ, Littlewood DTJ, editors. Animal Evolution: Genomes, Fossils, and Trees. Oxford: Oxford Univ Press; 2009. pp. 33–40.
-
- van den Biggelaar J, Dictus WJAG. Gastrulation in the molluscan embryo. In: Stern CD, editor. Gastrulation: From Cells to Embryo. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2004. pp. 63–78.
LinkOut - more resources
Full Text Sources
Other Literature Sources

