Three-Year Efficacy and Safety of a Silicone Oil-Filled Foldable-Capsular-Vitreous-Body in Three Cases of Severe Retinal Detachment
- PMID: 26855843
- PMCID: PMC4736667
- DOI: 10.1167/tvst.5.1.2
Three-Year Efficacy and Safety of a Silicone Oil-Filled Foldable-Capsular-Vitreous-Body in Three Cases of Severe Retinal Detachment
Abstract
Purpose: We previously designed a novel foldable capsular vitreous body (FCVB) to treat severe retinal detachment and evaluated its performance in a 1-year follow up study. The purpose of this study was to determine the efficacy and safety of a silicone oil (SO)-filled FCVB in a 3-year follow-up.
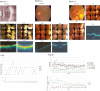
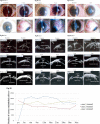
Methods: Standard three-port pars plana vitrectomy was performed, and the FCVB was triple folded and implanted in the vitreous cavity of three eyes. The SO then was injected into the capsule to support the retina. The eyes were examined using Goldmann applanation tonometry, fundus photography, optical coherence tomography (OCT), noncontact specular microscopy, and ultrasound biomicroscopy over a 3-year implantation period.
Results: At the 3-year follow-up, retinal reattachment was achieved in all three cases, with steady intraocular pressure. The visual acuity showed slight fluctuations, and it was slightly increased compared to baseline. Optical coherence tomography revealed decreased retinal thickness and an altered retinal structure in the implanted eyes compared to the control eyes. No keratopathy, glaucoma, SO leakage, SO emulsification, or other apparent complications occurred during the observation period.
Conclusion: The SO-filled FCVB was effective and safe as a vitreous substitute in three eyes over a 3-year observation period.
Translational relevance: Silicone oil emulsification is a severe complication after retinal detachment surgery. On the basis of animal experiments, we investigated a new strategy and product, the FCVB, to overcome this complication. In this pilot study, FCVB limited SO emulsification and migration. This study could lay the foundation for a further multicenter clinical trial.
Keywords: foldable capsular vitreous body; retinal detachment; silicone oil; vitreous substitute.
Figures

Similar articles
-
Study on the effectiveness and safety of Foldable Capsular Vitreous Body implantation.BMC Ophthalmol. 2019 Dec 18;19(1):260. doi: 10.1186/s12886-019-1268-x. BMC Ophthalmol. 2019. PMID: 31852464 Free PMC article.
-
Study on the efficacy and safety of foldable capsular vitreous body in the severe retinal detachment eyes.BMC Ophthalmol. 2022 Dec 15;22(1):491. doi: 10.1186/s12886-022-02729-9. BMC Ophthalmol. 2022. PMID: 36522622 Free PMC article.
-
Preliminary efficacy and safety of a silicone oil-filled foldable capsular vitreous body in the treatment of severe retinal detachment.Retina. 2012 Apr;32(4):729-41. doi: 10.1097/IAE.0b013e31822b1f80. Retina. 2012. PMID: 22105508
-
Foldable capsular vitreous body indications, complications, and outcomes: a systematic review.Graefes Arch Clin Exp Ophthalmol. 2023 Aug;261(8):2103-2116. doi: 10.1007/s00417-023-05995-5. Epub 2023 Feb 16. Graefes Arch Clin Exp Ophthalmol. 2023. PMID: 36795160
-
Advances in Polysaccharide- and Synthetic Polymer-Based Vitreous Substitutes.Pharmaceutics. 2023 Feb 8;15(2):566. doi: 10.3390/pharmaceutics15020566. Pharmaceutics. 2023. PMID: 36839888 Free PMC article. Review.
Cited by
-
Risk Factors for Trauma-Related Eviscerations: Analysis of 821 Cases.J Ophthalmol. 2019 Nov 11;2019:6198368. doi: 10.1155/2019/6198368. eCollection 2019. J Ophthalmol. 2019. PMID: 31827911 Free PMC article.
-
Consensus on the Treatment of Severe Ocular Trauma and Silicone Oil-Dependent Eyes Using Foldable Capsular Vitreous Body.J Evid Based Med. 2025 Jun;18(2):e70041. doi: 10.1111/jebm.70041. J Evid Based Med. 2025. PMID: 40571677 Free PMC article.
-
Study on the effectiveness and safety of Foldable Capsular Vitreous Body implantation.BMC Ophthalmol. 2019 Dec 18;19(1):260. doi: 10.1186/s12886-019-1268-x. BMC Ophthalmol. 2019. PMID: 31852464 Free PMC article.
-
Study on the efficacy and safety of foldable capsular vitreous body in the severe retinal detachment eyes.BMC Ophthalmol. 2022 Dec 15;22(1):491. doi: 10.1186/s12886-022-02729-9. BMC Ophthalmol. 2022. PMID: 36522622 Free PMC article.
-
Evaluation of the long-term effect of foldable capsular vitreous bodies in severe ocular rupture.Int J Ophthalmol. 2021 Dec 18;14(12):1935-1940. doi: 10.18240/ijo.2021.12.19. eCollection 2021. Int J Ophthalmol. 2021. PMID: 34926211 Free PMC article.
References
-
- Le GM,, Bishop PN. Adult vitreous structure and postnatal changes. Eye (Lond). 2008; 22: 1214–1222. - PubMed
-
- Quiram PA,, Gonzales CR,, Hu W,, et al. Outcomes of vitrectomy with inferior retinectomy in patients with recurrent rhegmatogenous retinal detachments and proliferative vitreoretinopathy. Ophthalmology. 2006; 113: 2041–2047. - PubMed
-
- Pastor JC. Proliferative vitreoretinopathy: an overview. Surv Ophthalmol. 1998; 43: 3–18. - PubMed
-
- Karel I,, Kalvodova B. Long-term results of pars plana vitrectomy and silicone oil for complications of diabetic retinopathy. Eur J Ophthalmol. 1994; 4: 52–58. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

