Clinical and Immunological Features of Opsoclonus-Myoclonus Syndrome in the Era of Neuronal Cell Surface Antibodies
- PMID: 26856612
- PMCID: PMC5823978
- DOI: 10.1001/jamaneurol.2015.4607
Clinical and Immunological Features of Opsoclonus-Myoclonus Syndrome in the Era of Neuronal Cell Surface Antibodies
Abstract
Importance: Most studies on opsoclonus-myoclonus syndrome (OMS) in adults are based on small case series before the era of neuronal cell surface antibody discovery.
Objective: To report the clinical and immunological features of idiopathic OMS (I-OMS) and paraneoplastic OMS (P-OMS), the occurrence of antibodies to cell surface antigens, and the discovery of a novel cell surface epitope.
Design, setting, and participants: Retrospective cohort study and laboratory investigations of 114 adult patients with OMS at a center for autoimmune neurological disorders done between January 2013 and September 2015.
Main outcomes and measures: Review of clinical records. Immunohistochemistry on rat brain and cultured neurons as well as cell-based assays were used to identify known autoantibodies. Immunoprecipitation and mass spectrometry were used to characterize novel antigens.
Results: Of the 114 patients (62 [54%] female; median age, 45 years; interquartile range, 32-60 years), 45 (39%) had P-OMS and 69 (61%) had I-OMS. In patients with P-OMS, the associated tumors included lung cancer (n = 19), breast cancer (n = 10), other cancers (n = 5), and ovarian teratoma (n = 8); 3 additional patients without detectable cancer were considered to have P-OMS because they had positive results for onconeuronal antibodies. Patients with I-OMS, compared with those who had P-OMS, were younger (median age, 38 [interquartile range, 31-50] vs 54 [interquartile range, 45-65] years; P < .001), presented more often with prodromal symptoms or active infection (33% vs 13%; P = .02), less frequently had encephalopathy (10% vs 29%; P = .01), and had better outcome (defined by a modified Rankin Scale score ≤ 2 at last visit; 84% vs 39%; P < .001) with fewer relapses (7% vs 24%; P= .04). Onconeuronal antibodies occurred in 13 patients (11%), mostly Ri/ANNA2 antibodies, which were detected in 7 of 10 patients (70%) with breast cancer. Neuronal surface antibodies were identified in 12 patients (11%), mainly glycine receptor antibodies (9 cases), which predominated in P-OMS with lung cancer (21% vs 5% in patients with OMS without lung cancer; P = .02); however, a similar frequency of glycine receptor antibodies was found in patients with lung cancer without OMS (13 of 65 patients [20%]). A novel cell surface epitope, human natural killer 1 (HNK-1), was the target of the antibodies in 3 patients with lung cancer and P-OMS.
Conclusions and relevance: Patients with I-OMS responded better to treatment and had fewer relapses than those with P-OMS. Older age and encephalopathy, significantly associated with P-OMS, are clinical clues suggesting an underlying tumor. Glycine receptor antibodies occur frequently in P-OMS with lung cancer, but the sensitivity and specificity are low. The HNK-1 epitope is a novel epitope in a subset of patients with P-OMS and lung cancer.
Conflict of interest statement
Figures

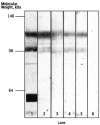

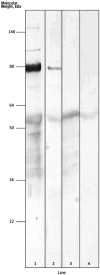
Comment in
-
New Findings in Adult Opsoclonus-Myoclonus Syndrome.JAMA Neurol. 2016 Apr;73(4):381-2. doi: 10.1001/jamaneurol.2015.4757. JAMA Neurol. 2016. PMID: 26856251 No abstract available.
-
Opsoclonus-Myoclonus Syndrome in the Era of Neuronal Cell Surface Antibodies: A Message for Clinicians.JAMA Neurol. 2016 Jul 1;73(7):891. doi: 10.1001/jamaneurol.2016.1161. JAMA Neurol. 2016. PMID: 27159217 No abstract available.
-
Opsoclonus-Myoclonus Syndrome in the Era of Neuronal Cell Surface Antibodies-Reply.JAMA Neurol. 2016 Jul 1;73(7):891. doi: 10.1001/jamaneurol.2016.1164. JAMA Neurol. 2016. PMID: 27159281 No abstract available.
References
-
- Caviness JN, Forsyth PA, Layton DD, McPhee TJ. The movement disorder of adult opsoclonus. Mov Disord. 1995;10(1):22–27. - PubMed
-
- Bataller L, Graus F, Saiz A, Vilchez JJ Spanish Opsoclonus-Myoclonus Study Group. Clinical outcome in adult onset idiopathic or paraneoplastic opsoclonus-myoclonus. Brain. 2001;124(pt 2):437–443. - PubMed
-
- Klaas JP, Ahlskog JE, Pittock SJ, et al. Adult-onset opsoclonus-myoclonus syndrome. Arch Neurol. 2012;69(12):1598–1607. - PubMed
-
- Pike M. Opsoclonus-myoclonus syndrome. Handb Clin Neurol. 2013;112:1209–1211. - PubMed
-
- Pranzatelli MR. The immunopharmacology of the opsoclonus-myoclonus syndrome. Clin Neuropharmacol. 1996;19(1):1–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

